HRV refers to the temporal changes in the beat-to-beat intervals in the heart, which is subject to continuous Autonomic Nervous System (ANS) and competing sympathetic versus parasympathetic control. HRV assessment has rapidly progressed in recent years from being predominantly a research-based tool to its translational use across several mainstream clinical and sporting applications [1-3]. These include the prevention of overtraining in relation to physical performance and in the management of mental stress [1,2,4-6]. The miniaturisation and increased portability of HRV equipment with the availability of smartphone-based platforms have significantly helped in this regard.
There is now a plethora of available HRV measures of varying complexity, however it is some of the short-term (≤5 minutes) measures that have provided the greatest use in day-to-day practice [7,8]. One of the most utilised HRV parameters is the RMSSD (root mean square of successive differences), which can be obtained from ultra short beat-to-beat recordings of less than two minutes [9-11]. It is thought to be a non-invasive surrogate for parasympathetic activity and vagal tone [7].
One of the areas of novel clinical interest in HRV assessment has been in the field of High Altitude (HA) medicine. HA related hypoxia leads to a compensatory rise in respiratory rate and tidal volume, known as the hypoxic ventilatory response, which acts to preserve tissue oxygenation [8,9]. This physiological response may be of considerable practical importance in relation to HRV measurement at HA, given the recognised influence of respiratory rate and tidal volume (i.e., minute ventilation) on HRV and its measurement [12,13].
An area of ongoing debate is the issue of whether controlled/paced breathing during HRV measurement generates more reliable HRV data than with relaxed and regular spontaneous breathing [14-18]. The comparative effects of spontaneous versus paced breathing on HRV have never been examined at HA. Establishing their potential influence on HRV at HA is a crucial methodological consideration. It is not unreasonable to hypothesize that by enforcing a paced breathing pattern genuine changes in HRV appreciated with the physiological hyperventilation of HA, could be obscured [19]. Furthermore, the ventilatory influence of HA-related illnesses such as HA Pulmonary Oedema (HAPE) and AMS on HRV could also be potentially mitigated by the use of paced breathing during HRV measurement. This issue assumes even greater importance, given several recent publications suggesting a potential link between changes in HRV and AMS development [20-22]. The published HRV at HA studies to date have mainly utilised spontaneous breathing during HRV measurement and the comparative effects of spontaneous versus paced breathing has not been examined [20-25].
In the present study we aimed to assess, for the first time, the effect of spontaneous versus paced breathing on HRV at HA as well as the influence of AMS on their level of this agreement.
Materials and Methods
This was a prospective observational study conducted over nine days on 30 healthy British military servicemen trekking in the Bernese Alps in Switzerland from the 18th to the 26th June 2017. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study. Baseline data was collected at 800 m (Basecamp; days 0-1). On day 2 the participants moved by road to 1200 m then on foot to Blumlisalphutte (2840 m) over four hours carrying a weight of 15 kg. There they spent three days (days 2-5) with training serials on a nearby glacier before returning to basecamp (day 5). At basecamp the subjects were split into three groups. Team 1 (n=9) remained at Basecamp (800 m) until day 9 (pm). Team 2 (n=12) went to Mönchsjoch hut (3658 m) by train and then light trek over the last one hour. From there they climbed to the 4107 m over days 5-8 before returning to Basecamp (day 8) where they stayed till the end of the data collection (day 9). Team 3 (n=9) travelled by road followed by a four hour trek to 2543 m. From there they climbed to 3583 m on days 5-8, before returning to basecamp (days 8-9). Measurement of HA-symptoms, HRV and heart rate were measured twice daily. Saboul D et al., observed significant differences in short term daily measures of HRV including RMSSD and heart rate between spontaneous and controlled breathing in 10 healthy subjects [12]. Based on this data and an expected correlation between pairs of >0.70 we calculated that at sample size of >20 subjects measured twice daily over at least seven days (>280 paired samples) would have >90% power to detect a mean difference in HRV score of ≥1.5.
Physiological and Physical Assessments
HA-related symptoms were recorded using the Lake Louis Scoring (LLS) system. AMS was defined as LLS of ≥3 in the presence of headache and a recent altitude gain [26,27]. The Borg rating of perceived exertion was recorded at the end of each day. This is a 15 point numerical scale numbered from 6-20, with values of 6 representing the resting state and 20, exhaustive exercise (Borg 1970) that has been used at HA previously [28]. The highest Rating of Perceived Exertion (RPE) during the day was recorded to reflect the overall effort [28].
Assessment of Heart Rate Variability
This was performed twice daily post micturition and prior to breakfast/dinner or caffeine. Conditions were kept consistent with previous studies of HRV at HA with all subjects being seated in a covered environment, wearing warm clothing for at least five minutes before the HRV recordings were obtained [23,24]. HRV variability was obtained using a finger sensor attached to mobile phone (AppleTM iphone 6s) installed with the ithlete HRV app (HRV Fit Ltd. Southampton, UK) as previously described and validated [23,25,29,30]. Two consecutive 55 second HRV recordings were obtained separated by a one minute wash out rest period. This validated time period is set by the device and cannot be altered. The first HRV reading was undertaken with the subjects breathing spontaneously, after five minutes of relaxation. The second HRV measurement was recorded during paced breathing at frequency of 7.5 breaths per minute. This guided breathing protocol within the ithlete mobile phone app is delivered via visual onscreen prompts to guide the speed and duration of both inspiration and expiration [23,25,29,30].
The ithleteTM HRV score modifies the acquired RMSSD (root mean squared of successive differences) by taking the natural log transformation and multiplying by twenty (lnRMSSD×20). This provides a more interpretable figure for the user on a ~100 point scale [29,30]. In a previous study of 12 healthy subjects studied over seven altitudes from the 1400-3600 m the coefficient of variation for paired HRV readings using paced breathing was 5.5% [23].
Statistical Analysis
Sample size calculations were performed using a proprietary determined sample-size calculator (GraphPad StatMate version 2.00 for Windows). Data was analysed using GraphPad InStat version 3.05 and with all graphical figures presented using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com). Data inspection and the Kolmogorov-Smirnov test was undertaken to assess normality of all continuous data. Results are presented as mean±Standard Deviation (SD). Paired comparisons of parametric and non-parametric continuous data were assessed using a paired t test and a Wilcoxon matched pairs test and their correlation using Pearson and Spearman correlation coefficients ±95% Confidence Interval (CI) respectively. Only correlations with an R>0.20 were reported. An F-Test was performed to assess whether any potential differences in standard deviations in HRV scores between spontaneous versus paced breathing were significant.
The accuracy of agreements in HRV scores between that obtained from spontaneous versus paced breathing were assessed using Bland-Altman plots [23,31] in which the difference between the two values were compared with the average values from the comparative two readings. The bias was defined as the mean±SD of the difference between the readings. Reasonable agreement was defined as <5% of readings being within 1.96 SD (95% CI) from the mean.
A factorial repeated measures ANOVA was undertaken to assess the main effects of altitude and breathing (spontaneous vs paced) on HRV scores and any potential interactions of altitude and breathing method on the paired differences in HRV Scores. A p-value of <0.05 was considered significant for all comparisons.
Results
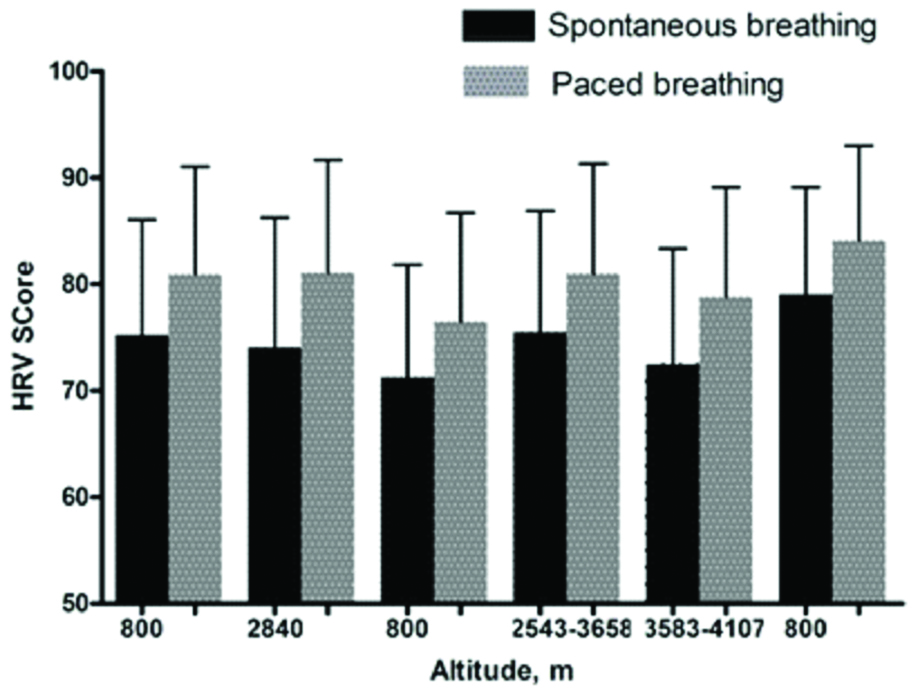
The average age of the 30 included participants was 33.3±7.7 years with an age range of 22-54 years. They were all caucasian and non-smokers with a mean body mass index of 26.0±2.3 kg/m2. There was a significant reduction in SpO2 and an increased in LLS and heart rate at higher altitudes of ≥2543 m [Table/Fig-1]. There was an inverse correlation between SpO2 and LLS (r=-0.25; -0.33 to -0.16: p<0.0001). The prevalence of AMS was 13.3% at 2840 m, 22.2% at 2543-3658 m and 41.7% at 3583-4107 m. The cases of AMS were generally mild (>90% LLS 3-5).
The effects of high altitude on HRV, physiological and mountain sickness scores.
| 800 m | 2840 m | 800 m | 2543-3658 m | 3583-4107 m | 800 m | p-value |
|---|
| HRV score |
| -Spontaneous breathing | 75.2±10.8 | 74.1±12.2 | 71.3±10.6 | 75.5±11.4 | 72.2±10.9 | 78.8±9.9 | 0.0006 |
| -Paced breathing | 80.9±10.1* | 81.1±10.6* | 76.5±10.2* | 81.0±10.3* | 78.8±10.3* | 84.1±8.9* | <0.0001 |
| Heart rate |
| -Spontaneous breathing | 65.4±8.5 | 70.1±11.3 | 72.9±10.6 | 69.3±8.6 | 70.6±8.4 | 65.1±10.1 | <0.0001 |
| -Paced breathing | 65.3±8.8 | 69.3±10.7 | 71.8±10.2 | 68.6±8.7 | 69.1±8.2 | 64.7±8.8 | <0.0001 |
| SpO2, | 95.9±2.6 | 91.9±3.0↑ | 96.0±1.8 | 89.8±3.3↑ | 89.9±4.3 | 96.6±2.2 | <0.0001 |
| Lake Louise score | 0.21±0.6 | 1.0±4.3 | 0.22±0.7 | 1.2±1.5 | 1.3±1.4 | 0.30±0.60 | <0.0001 |
↑Significance vs baseline on Post-test; *Significant paired differences between spontaneous vs paced breathing
There were a total of 511 paired HRV scores obtained. Overall, HRV scores obtained from spontaneous versus paced breathing strongly correlated (511 pairs; r=0.79: 0.75-0.82; p<0.0001) [Table/Fig-2]. This significant correlation was consistent at both lower (<2543 m) altitudes and higher altitudes (≥2543 m). There was significant inverse correlation between heart rate and the HRV score (r=-0.68; -0.73 to -0.63; p<0.0001). Borg RPE scores positively correlated with resting heart rate (r=0.26; 0.13 to 0.38; p<0.0001) and inversely with HRV score (r=-0.30; -0.42 to -0.17; p<0.0001) measured at the same time.
Linear regression (95% CI) of comparative HRV scores obtained with spontaneous versus paced breathing.
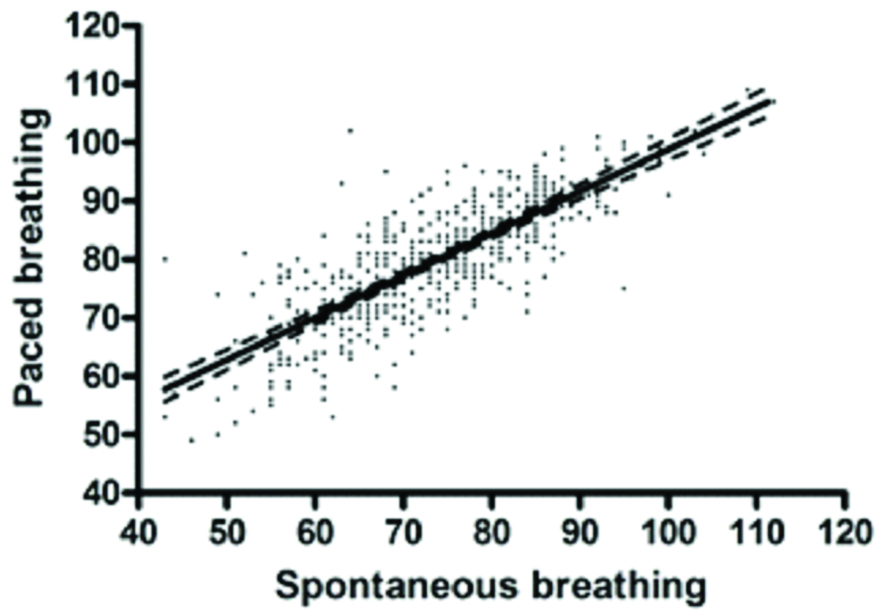
The HRV scores were consistently higher with paced versus spontaneous breathing (73.3±11.4 vs. 80.1±10.4; mean difference+6.1: p<0.0001) [Table/Fig-3]. The standard deviation around HRV scores was marginally, yet significantly higher, for spontaneous versus paced breathing (F=1.2; p=0.04). HRV scores were lower in those with vs without AMS for both breathing methods. However, this difference was only significant for spontaneous (74.3±11.4 vs. 68.1±12.1; p=0.03) but not paced breathing (80.3±10.4 vs. 76.3±11.8; p=0.13).
Comparison of relative HRV scores (mean±standard deviation) with each altitude for spontaneous versus paced breathing.
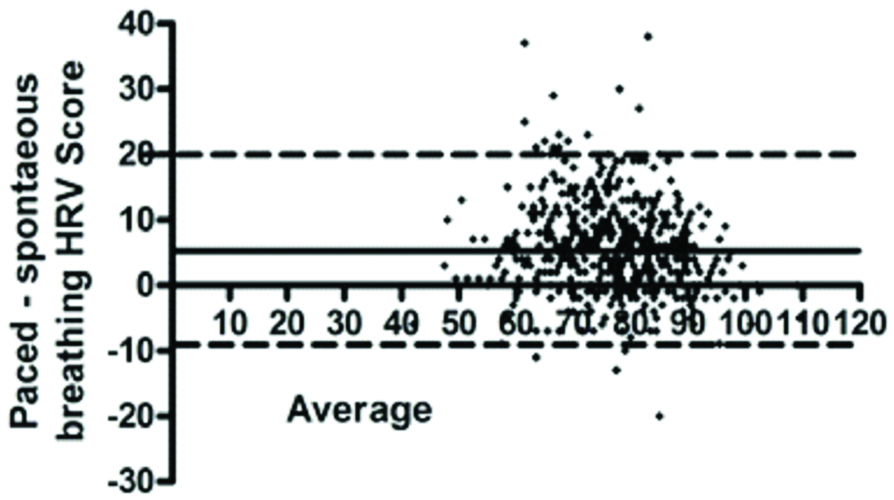
On Bland Altman analysis identified a strong level of agreement between HRV scores (511 pairs: 96.1% within the 95% limit of agreement) obtained with spontaneous versus paced breathing but with a consistent bias to higher scores with paced breathing {mean difference (bias) +6.00; 95% CI -8.0 to 20.1)} [Table/Fig-4]. This bias further increased when only those (n=15) with AMS were examined (93.8% within 95% limit of agreement; bias +8.0: 95% CI -5.5 to +22.0).
Comparison of HRV scores obtained by spontaneous versus paced breathing over all time points: differences (Y-axis) are compared with the average HRV score
There was a significant main effect for altitude (F=5.3; p<0.0001) and breathing (F=262.1; p<0.0001) on the paired HRV scores. However, there was no altitude-x-breathing interaction (F=1.2; p=0.30). For the dependent variable of heart rate there was a main effect for altitude (F=7.0; p<0.0001) and breathing (F=10.7; p=0.001) but again no altitude-x-breathing interaction (F=0.60; p=0.70).
Discussion
This is the first study to assess the comparative effects of paced versus spontaneous breathing on short term measures of HRV at sea level and HA. It was found that there was a strong agreement between the two methods. HRV scores were consistently higher, though less variable with PB. These differences increased with AMS. Whilst there was a significant main effect for altitude and method of breathing on HRV scores there was no overall interaction between altitude and breathing method on HRV scores. The RPE inversely correlated with HRV, with higher perceived exertion being linked to lower HRV.
The influence of ventilation on heart rate and HRV has been well investigated at sea level [11,12,16-18]. The concept behind using paced breathing during HRV assessment is that by standardising the breathing pattern (respiratory rate, tidal volumes, inspiratory and expiratory time) the inter-sampling variability between HRV measurements should be reduced and validity improved. Published comparative data on differences in HRV measurements between spontaneous versus paced breathing have yielded inconsistent results. This variability may, in part relate to relative differences in environmental conditions, the duration and frequency of paced breathing used and the HRV parameters examined [11,12,32]. These disparities probably explain why a consensus guideline on paced breathing during HRV measurement has not yet been established.
The effects of breathing method during HRV measurement at HA had not previously investigated, despite an increasing number of published studies in this environment, which was the impetus for this study. Increasing minute ventilation is one of the most consistent physiological effects of HA exposure and acclimatisation [7,8]. Hypoxic stimulation of arterial chemoreceptors leads to compensatory hyperventilation in order to limit the fall in alveolar PO2 and the degree of arterial hypoxaemia [7]. Despite these factors we still observed a strong correlation in the ithlete HRV scores (r=0.79) between spontaneous and paced breathing over several days at variable altitudes. This degree of correlation is remarkably similar to the comparative published sea level data (correlation coefficients of ≥0.70) [12].
The paced breathing rate generated by the ithlete app was fixed throughout the present study, whereas it was not controlled during spontaneous breathing. Respiratory rate and minute ventilation (respiratory rate xtidal volume) are known to rise in response to the worsening hypoxia at increasing HA [7,8]. It was anticipated that this would lead to greater relative differences in the minute ventilation between spontaneous and paced breathing at higher altitudes during HRV measurement: as whilst the spontaneous respiratory rate would expectedly increase the paced rate, would obviously remain unchanged at 7.5 breaths per minute. Given the recognised influence of respiratory rates on HRV, this should have translated into greater discordance in HRV scores between the two breathing modes at higher altitudes, yet this phenomenon was not observed. Although it was noted that a significant main effect for altitude and breathing modality (higher HRV score with paced breathing), there was no altitude-x-breathing interaction on HRV scores at HA. This would suggest that paced breathing does not appear to negate the HA related changes in the ithlete HRV score and RMSSD. However, given that the higher HRV scores with paced breathing, the two methods cannot be used interchangeably.
HRV scores tended to be lower with AMS (versus without) and inversely correlated with increasing RPE, which is an expected finding and consistent with previous research [21,22,33,34]. Nevertheless, it is interesting that whilst this difference was observed for both spontaneous and paced breathing this difference was only statistically significant for spontaneous breathing. This might be a chance effect or related to bias due to the smaller sample size for the AMS group. A further exploration of comparative HRV measurements at higher altitudes and with a greater AMS burden and severity is clearly warranted. This is needed to better determine whether paced breathing could act to mitigate some genuine changes in HRV with very HA and significant AMS, by the implementation a short period of forced and unnatural breathing at HA.
In the present study one measure was only assessed of HRV obtained over an ultra short recording time using the ithlete validated HRV protocol [10,23]. This device was specifically selected as it is battery operated (via the mobile phone charge), portable, user friendly and affordable which is ideal for HA research and maximises its translational potential [29,30]. Its HRV score is derived from the RMSSD which is strongly correlated with other time domain measures of HRV [7,23]. The ithlete HRV score is thought to reflect parasympathetic control and is thus highly responsive to acute changes in vagal tone and may be useful as a marker of HA acclimatisation [7,25,30]. It has been shown that HA acclimatisation is associated with changes in parasympathetic output and the withdrawal of cardiac vagal modulation has been implicated in AMS development [24,33].
The order of breathing in the protocol was fixed throughout out the present study with spontaneous breathing always preceding paced breathing. This sequence was chosen for two important reasons. Firstly this order is consistent with several other published comparative studies [16-19]. Secondly, this sequence is logistically easier and makes physiological sense as the first 55 second HRV measurement during spontaneous breathing was conducted immediately after five minutes of spontaneous breathing. It was felt that reversing this order could lead to negative bias. A parallel study of age matched groups randomised to spontaneous then fixed breathing and vice versa would have been ideal but would have required a larger sample size.
Limitation
This study has a number of limitations that should be acknowledged. The altitudes studied were modest, the incidence of AMS was relatively low and the majority of AMS were very mild (predominantly LLS 3-4), which may have limited the impact of the finding. It only measured one measure of short term HRV using the RMSSD derived ithlete HRV score obtained over only 55 seconds. Hence, applicability of these findings to other HRV measures obtained over a longer recording period (>1 minute) cannot be certain. We did not measure the comparative respiratory rates and tidal volumes of the two breathing strategies, hence, we do not know for certain what their true differences were.
Conclusion
Ithlete HRV score measured using spontaneous breathing was strongly correlated with that for paced breathing at moderate HA. However, the score was consistently and significantly higher and the variance lower with paced breathing. Whilst the relative differences in HRV with spontaneous and paced breathing were not affected by altitude, the presence of AMS may be an important confounder. Further data at higher altitude and with a greater of AMS would be useful to further explore this finding.
Compliance with Ethical Standards
Dr. Christopher J Boos, Department of Cardiology, Poole Hospital NHS Foundation Trust, Longfleet Rd. Poole, Dorset, BH15 2JB.
↑Significance vs baseline on Post-test; *Significant paired differences between spontaneous vs paced breathing