A pivotal target of contemporary dentistry is to manage non-cavitated lesions non-invasively through remineralization in an attempt to prevent disease progress and improve esthetics, strength and function. The oral cavity is a combat zone of activities of demineralization and remineralization. The ratio between demineralization and remineralization is vital in determining the hardness and strength of tooth structure. Demineralization results from a complex chemistry between bacteria, diet and salivary components releasing organic acids that causes a drop of pH leading to demineralization resulting in excessive loss of minerals, which in turn leads to enamel loss and cavitation. Conversely, remineralization occurs when the pH rises and there is deposition of calcium, phosphate and fluoride ions in the form of fluorapatite that is more resistant to acid dissolution. Therefore, it is cerebral that the best strategy for caries management is to focus on methods for improving remineralization.
Recently, a variety of remineralizing agents like fluorides, casein phosphopeptide, novamin etc., that aid in remineralizing the tooth structure are available commercially. These agents are part of a new era in dentistry that is aimed at improving the dental hard tissue with different modes of action, depending on the micro environment around the tooth. Fluoride has always been a cornerstone in dentistry. But, its ability to promote net remineralization is limited by the availability of calcium and phosphate ions [1]. Fluoride ions can drive the remineralization process only if there is adequate supply of salivary calcium and phosphate [2]. Hence on topical application of fluoride, availability of calcium and phosphate can be a limiting factor. Therefore, Clinpro tooth crème was introduced that contained 0.21% sodium fluoride and functionalized tricalcium phosphate. GC tooth mousse (casein phosphopeptide amorphous calcium phosphate) was added into preventive dentistry with the promise to modulate bioavailability of calcium phosphate levels by maintaining ionic phosphate and calcium super saturation to increase remineralization [3]. In addition CPP-ACP has shown to reduce the streptococcus mutans biofilm development on GIC and also disrupt the established biofilms on enamel [4]. Novamin (calcium sodium phosphosilicate-Shy-NM) is a material containing bioactive glass which belongs to the class of highly biocompatible materials that were originally developed as bone regenerative materials. These materials are reactive when exposed to body fluids and deposit hydroxyl carbonate apatite, a mineral chemically similar to natural tooth mineral [5].
Literature search showed all the three agents to be effective in remineralizing, inspite of differences in composition and mechanism of action. Hence, this study was conducted to compare the ability of three commercially available agents in remineralizing enamel in an in vitro condition, assessed by SMH and SEM-EDX analysis.
Materials and Methods
Informed consent–Institutional ethical clearance was obtained for this study; however, human subjects were not a part of this work.
Specimen Conditioning and Storage
Sixty human premolars extracted for orthodontic reasons were selected for the study. The teeth included were free from dental caries, restorations and developmental defects. The samples were cleaned of calculus and soft tissues and stored in 10% formalin prior to the start of the study.
Sample Preparation and Study Design
The sixty teeth were randomly divided into 3 groups of 20 each. The groups were named as follows:
Group A-GC tooth mousse (CPP-ACP)
Group B-Clinpro (sodium fluoride with f-TCP)
Group C-Shy–NM (Novamin)
In order to section the teeth, each sample was stabilised in IsometTM 5000 linear precision saw which is designed for cutting various material types with minimal deformation. The teeth were sectioned horizontally at the level of the cemento enamel junction, separating the crown and root parts of the tooth. The root portion was discarded. Later, the coronal sections of the tooth were further divided buccolingually and mesiodistally into 4 blocks. Three blocks of each tooth were selected as the test samples, as separate pieces were needed to undergo baseline, demineralization and remineralization assessment. The fourth block was henceforth discarded.
Following this, the buccal or lingual surface of each block was flattened and polished using 200-800 available grit abrasive papers. These were subsequently mounted in self cure acrylic resin with either surfaces facing upward and exposed. The acrylic resin blocks were made with moulds of the dimension (10×10 mm) to standardise the study [6]. The specimens were then stored in artificial saliva. The artificial saliva was replaced on a 24 hour period. The samples were then subjected to Vickers hardness testing and SEM-EDX. Ethical approval for this study was obtained from the Institutional Committee for ethical research.
Demineralization of the Samples
The samples were demineralized using freshly prepared McInnes demineralizing solution which consisted of 1 mL of 36% hydrochloric acid, 1 mL of 30% hydrogen peroxide and 0.2 mL of anesthetic ether mixed in the ratio of 5:5:1. The freshly prepared demineralizing agent was applied to the enamel surface using a cotton applicator. The demineralization was carried out in two cycles with an application time of minimum 3 minutes at 24 hour interval. After demineralization the samples were washed in running water, damped dry and subjected to surface microhardness testing and SEM-EDX.
Remineralization of the Sample
After demineralization, the samples of each group were subjected to a 30 days remineralization using their respective toothpastes. The paste was applied with cotton applicator tips, 3 minute twice daily for 30 consecutive days and stored in artificial saliva. At the end of 30 days the samples were retested for microhardness and SEM-EDX.
Vickers Microhardness Testing
In Vickers hardness testing the hardness was calculated by the formula:
VHN (Vickers Hardness Number)=964.L.lv-2 where, L-load; lv-depth of indentation
The test specimens were placed on the stage of the tester and stabilised. The test was carried out where the indentations were made with a rate of 2.942 N force/14 sec. The indentation formed was viewed and measured on the display monitor with 10x objective lens. To avoid discrepancy from the curvature of enamel surface, the average microhardness of the specimens were determined from five indentations.
Energy Dispersive X-Ray Analysis
The Scanning Electron Microscope (SEM) was used for the analysis of surface topographies and fracture morphology of metallic and non metallic structures. EDX has been used for elemental analysis at the ultra structural level. It is a microanalytical technique that was used in conjunction with the SEM wherein SEM does the structural analysis and elemental analysis was done by EDX.
Results
All the results were calculated as mean±standard deviation using SPSS software version 20.0 (SPSS Inc, Chicago, USA). One-way ANOVA (Kruskal Wallis test) was used to compare the results of all the three groups p<0.05 was considered significant [Table/Fig-1].
Statistical intergroup comparison of the three agents. As there was no significant difference found between CPP-ACP and Novamin, comparison of groups, showing a significant p-value, is tabulated.
| Groups | p-value |
|---|
| Sodium Fluoride | CPP-ACP | 0.019* |
| Novamin | 0.029* |
p<0.05 is considered significant
Vickers Hardness Testing
The microhardness of the baseline, demineralized and remineralized samples were recorded. It was observed that after 48 hours of demineralization, there was a significant decrease in microhardness of all the samples from the baseline values. The specimens treated with sodium fluoride showed the highest microhardness value (274.83±13.62) followed by CPP-ACP (266.85±37.80) and Novamin (257.64±27.72) as given in [Table/Fig-2]. The level of significance between the microhardness of remineralized enamel in three groups were compared using Multiple Comparison Dun-Bonferroni test and the results showed that there was significant difference in VHN between:
Mean surface microhardness (VHN) of baseline, demineralized and remineralized sample.
| Agents | Baseline | Demineralized | Remineralized |
|---|
| CPP-ACP | 263.11±24.13 | 154.52±16.41 | 266.85±37.80 |
| Sodium Fluoride | 258.58±13.43 | 106.64±18.67 | 274.83±13.62 |
| Novamin | 252.28±18.24 | 159.24±21.03 | 257.64±27.72 |
(Mean±SD; n=20)
Group C (Novamin) and Group B (Clinpro)
Group A (CPP ACP) and Group B (Clinpro) [Table/Fig-1]
But there was no significant difference in microhardness between group A and C indicating that the quality of the remineralized enamel was superior for sodium fluoride.
SEM-EDX
SEM images of the baseline enamel under 5000x magnification showed a smooth enamel texture without noticeable surface porosities indicating normal enamel as demonstrated in [Table/Fig-3a]. In contrast, the demineralized enamel was disorganised, with variable sizes of porosities viewed all over the surface as seen in [Table/Fig-3b]. On EDX analysis, the values of demineralized enamel were found to have lower calcium phosphate than the baseline values.
SEM images. a) SEM image of baseline enamel; b) SEM image of demineralized enamel. (5000x)

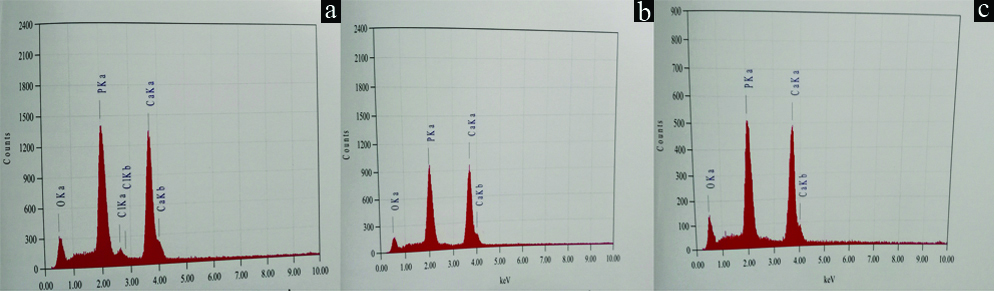
After remineralization, the surface morphology was seen to be of the highest quality in samples treated with Novamin and Sodium fluoride. They resembled normal enamel, when compared to the image of CPP-ACP which had localised porosity and uneven surface texture [Table/Fig-4a-c]. The elemental analysis showed an increase in calcium and phosphate ratio in all three groups, with Novamin and sodium fluoride having highest values [Table/Fig-5,6].
SEM images after remineralization. a) SEM image of CPP-ACP; b) SEM image of Clinpro; c) SEM image of Novamin. (Images from left to right)

Elemental analysis after remineralization. a) Elemental ratio of CPP ACP; b) Elemental ratio of Clinpro; c) Elemental ratio of Novamin. (Left to right)
Calcium/Phosphorus ratio of baseline, demineralized and remineralized samples.
| Groups | Baseline | Demineralized | Remineralized |
|---|
| A | 2.72 | 2.31 | 2.39 |
| B | 2.74 | 2.35 | 2.45 |
| C | 2.75 | 2.36 | 2.69 |
Discussion
Modern prospective caries research studies focus on bringing out small changes in the mineral content of the teeth enabling them to withstand strong demineralizing challenges. One of the recent advancement is the introduction of remineralizing toothpastes into the market, which on daily use claims to combat the lost ions from the teeth undergoing demineralization thereby preventing caries attack. The early non invasive intervention of an active carious lesion into an inactive state forms the basis of preventive dentistry [3].
The results of the present study will be beneficial to a caucus of pediatric and preventive dental practitioners, who could direct their patients on using the premier remineralizing agent currently and also implement them into clinical practice. It can help in mineralization and management of hypocalcified lesions, desensitization of exposed dentine affected by dental erosion and after debonding of brackets in lieu of completion of orthodontic treatment [5]. Therefore the present study was undertaken to compare the efficiency of three latest remineralizing dentifrice namely, ClinproTM (5000 ppm sodium fluoride with F TCP), Shy-NMTM (bioactive glass novamin) and GC Tooth mousse TM (CPP-ACP).
It was ineluctable to compare the nature of remineralized enamel in terms of their quality and quantity therefore; two parameters were considered in this study, hardness and surface property with elemental analysis. Hardness was measured with Vickers Hardness tester and surface property with SEM in conjunction with EDX. The microhardness testing showed that, all the three groups showed a decrease in microhardness from baseline values after demineralization that subsequently increased after remineralization. On individual comparison, sodium fluoride with F TCP had more SMH than bioactive glass and CPP-ACP. These results were contradictory to previous studies which demonstrated that CPP-ACP had more efficiency that fluoride in remineralizing enamel samples [7,8]. The reason for this variation could be attributed to the fact that both above mentioned studies had used 500 ppm and 1100 ppm fluoride respectively whereas in this study 5000 ppm fluoride was used and toothpastes containing 5000 ppm fluoride has been already proved to be more efficient than other concentrations i.e., 2800 ppm and 1450 ppm of fluorides [9]. Also, daily use of fluoridated toothpaste with 5000 ppm fluoride was proved to be more superior to CPP-ACP [10].
Another likely reason for this difference could be due to the use of Clinpro toothpaste. Clinpro toothpaste is superior as it contains a combination of sodium fluoride with functionalized tricalcium phosphate (f-TCP) which provides a continuous reservoir of ions. Functionalized tricalcium phosphate, not only ensures a controlled supply of calcium and phosphate ions, but also enhances the action of fluoride on enamel surfaces. While other calcium phosphate additives may require an acidic pH, TCP can offer optimal benefits when delivered in a neutral pH environment [11]. Therefore, TCP ingredient can enhance mineralization and help build high quality, acid resistant mineral without the need for high levels of calcium [12]. Two other previous studies that compared Clinpro with CPP-ACP reported that Clinpro that contained 5000 ppm sodium fluoride with TCP was superior to CPP-ACP and varying concentrations of fluoride [13,14]. Therefore it could be said that CPP-ACP favours remineralization although the properties are inferior to sodium fluoride.
On comparing CPP-ACP and Novamin, previous studies have shown that Novamin had greater SMH than CPP-ACP and was slightly superior to sodium fluoride [15]. However, when sodium fluoride and Novamin were compared, Novamin was found to be inferior in the present study. However, studies by Vahid Golpayegani M et al., and Gjorgievska ES et al., both of which compared Novamin and sodium fluoride reported that Novamin had a higher capability to enhance enamel resistance than sodium fluoride due to the ability of Novamin to increase the fluoride uptake of teeth [16,17]. In the study by Manoharan V et al., remineralization potential was found to be better with CPP-ACFP than Novamin. The probable reason could be due to the presence of additional fluoride in CPP-ACP which is known to provide a synergistic effect in remineralization [18].
When the enamel surface topography in SEM images were evaluated, it was observed that samples remineralized with Novamin (Bioactive glass) displayed an image that was very uniform and there was nearly complete repair of porosities when compared to the other two groups. The EDX images also showed a well defined uniform protective layer on the enamel surface without any porosity. This image more or less resembled natural enamel surface.
The image of Clinpro samples also suggested the surface morphology to be similar to that of normal enamel, although localised porosities were still observed. The surface property of Clinpro was however inferior to that of novamin, but superior to CPP-ACP, which showed only a partial repair of porosities and the uniformity of the surface was hardly distinguishable suggesting variation in remineralization.
On comparing the calcium phosphate ratios using SEM-EDX of all the three groups, it was observed that the Ca/P ratio fell to a considerable level in all the groups after demineralization and there was significant increase in the mineral content from demineralized enamel to remineralized in all three groups. However, these values were just below the baseline values in all three groups. This is similar to an earlier study by Rao A et al., who compared the Ca/P values of sodium fluoride (331.74±5.78) and nanohydroxyapatite (314.83±1.91) after remineralization and found that the remineralized values of both groups did not reach the baseline values of (343.29±5.74), (339.22±3.22) respectively [19].
In the present study, it was found that the recovery of mineral content was more in novamin group compared to Clinpro and CPP-ACP group. This could be because ions from bioactive glass release hydroxycarbonate apatite (HCA) particles. These particles attach to tooth surface and continue to release ions and remineralize the tooth surface even after initial application. An earlier study performed by Wang Z et al., in 2011 that showed, application of Novamin toothpaste for 7 days was responsible for the production of a smear layer-like coating that occluded most of the tubule orifices [20]. The results were also consistent with the study done by Gjorgievska ES et al., who concluded that treatment of demineralized teeth with toothpastes containing hydroxyapatite or bioactive glass resulted in repair of the damaged tissue. A protective layer of deposits was found to be formed on the enamel surface [17]. However, in contrast, the use of the toothpaste with 1040 ppm fluoride as NaF did not lead to any observable remineralization. Other studies comparing the SEM-EDX values of samples treated with bioactive glass, CPP-ACP and fluoride found that bioactive glass had similar results stating that bioactive glass showed the maximum remineralization [21,22]. Therefore, within the limits of this study it could be said that enamel treated with Sodium fluoride plus f-TCP was qualitatively and quantitatively superior.
Limitation
Being an in vitro trial, this study could not exactly simulate the oral environment despite using artificial saliva as the storage media. The influence of host factors could not be predicted. Studies involving electrophoresis and pH Cycling could influence the effect on demineralized enamel. This is one of the drawbacks of the study [23]. Therefore, further in vivo studies with large sample size may be required to warrant the results.
Conclusion
Based on the results of this study, it has been demonstrated that sodium fluoride containing toothpaste can significantly remineralize the enamel and promote regression of demineralization. Furthermore, the quality of remineralized enamel in terms of surface microhardness was superior in enamel treated with fluoride than with CPP-ACP and equivalent to that of bioactive glass. Therefore, within the limits of the above conducted invitro study it can be concluded that calcium phosphate based remineralizing technology and bioactive glass look promising as an adjunctive treatment to topical sodium fluoride in the non invasive management of early carious lesion, but not as an alternative.
p<0.05 is considered significant