Colonic Metastasis of Renal Cell Carcinoma with Sarcomatoid Differentiation
D’Sa Bouvier Francis Valere1, Nazima Haider2, Sohaila Fatima3
1 Specialist, Department of Pathology, Aseer Central Hospital, Abha, Aseer, Saudi Arabia.
2 Assistant Professor, Department of Pathology, King Khalid University, Abha, Aseer, Saudi Arabia.
3 Lecturer, Department of Pathology, King Khalid University, Abha, Aseer, Saudi Arabia.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nazima Haider, Tabjiya, Abha, Aseer, Saudi Arabia.
E-mail: nazima_haider@yahoo.com
Renal Cell carcinoma (RCC) can metastasize to any site in the body but metastasis to Gastrointestinal Tract (GIT) is extremely rare. Sarcomatoid RCC (sRCC), an aggressive subclone can be seen in variable proportions with other histological subtypes of RCC and is metastatic in majority of the cases. However, sRCC in association with primary papillary carcinoma metastasizing to colon is very rare, to the best of authors’ knowledge has never been reported before. Colonic metastasis of RCC is challenging for both the clinician and pathologist. We hereby present a case of 33-year-old female, a known patient of renal cell carcinoma with left nephrectomy done 3 years back, who presented with a descending colonic mass.
Gastrointestinal tract, Metastasis, Primary papillary carcinoma
Case Report
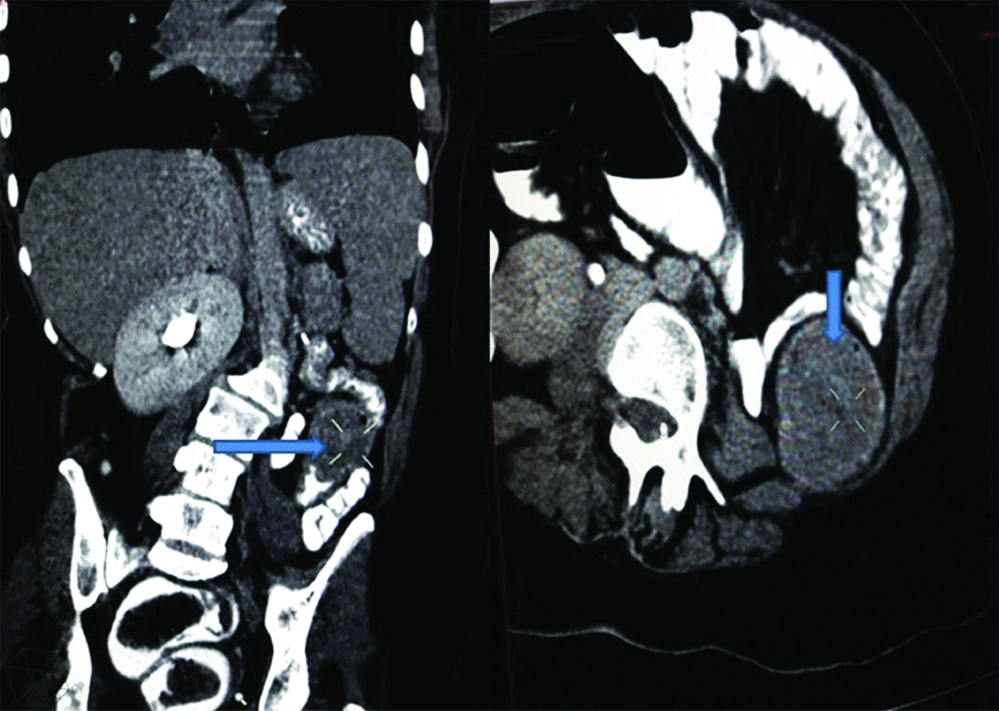
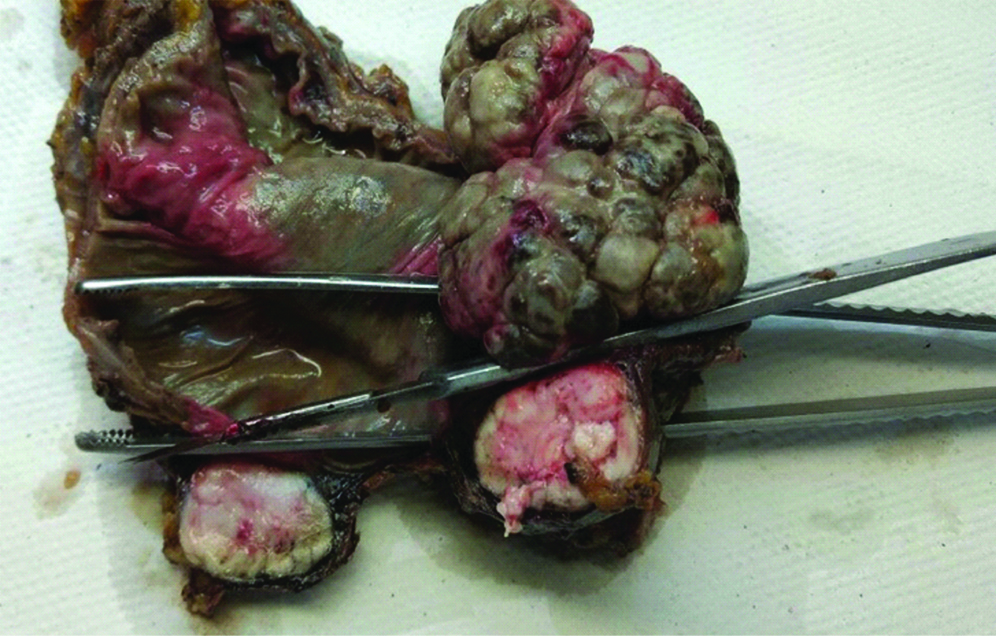
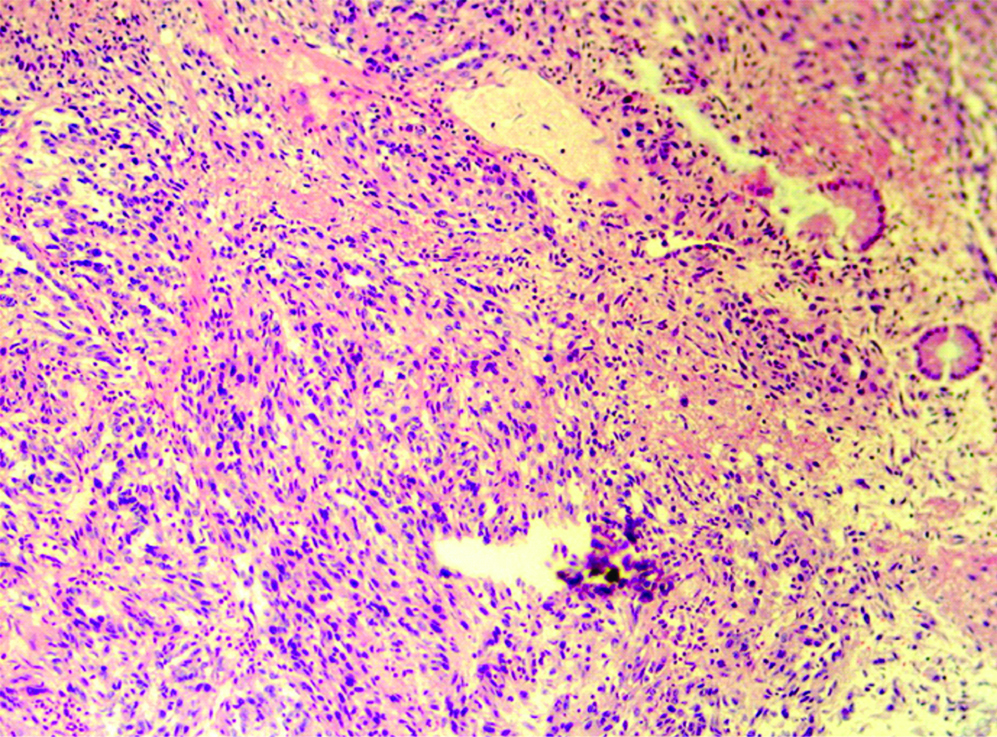
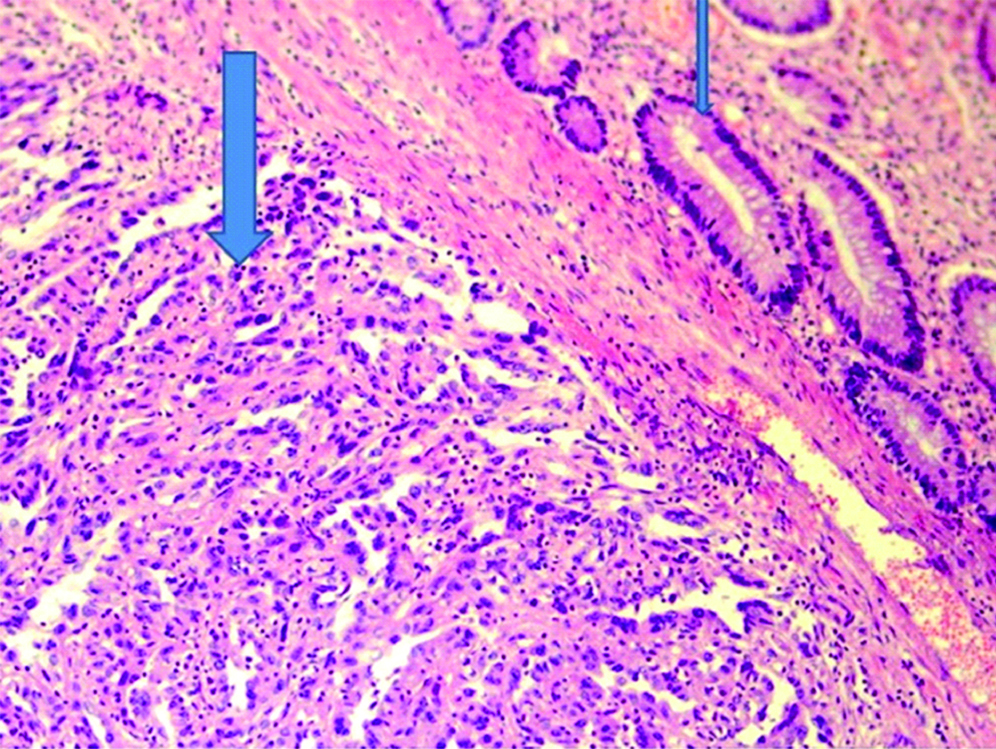
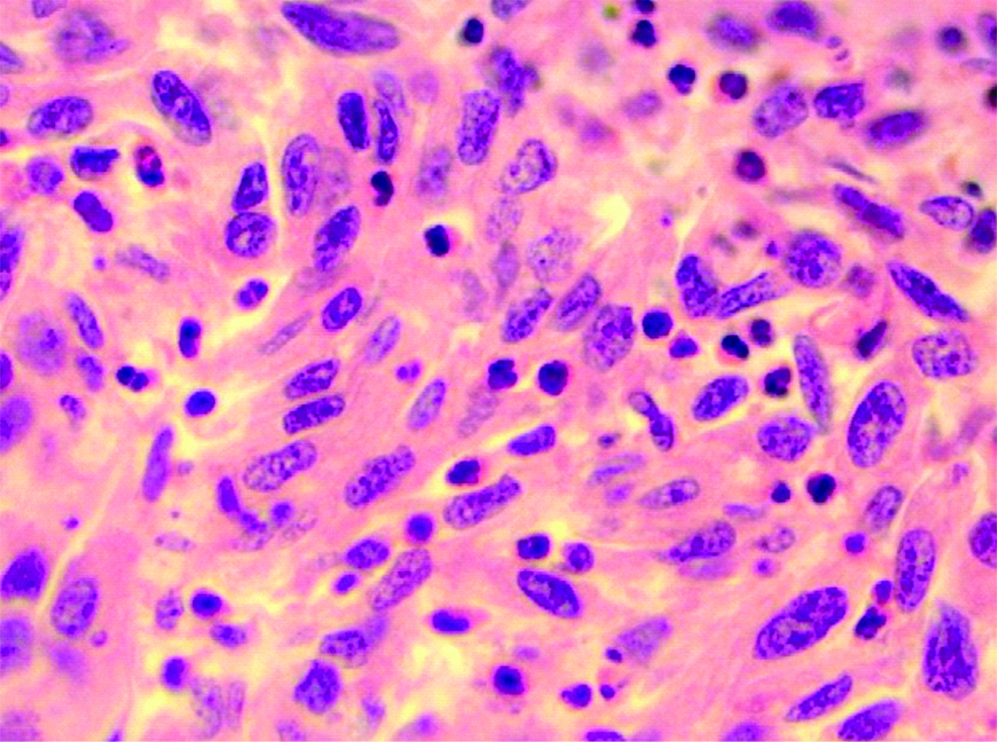
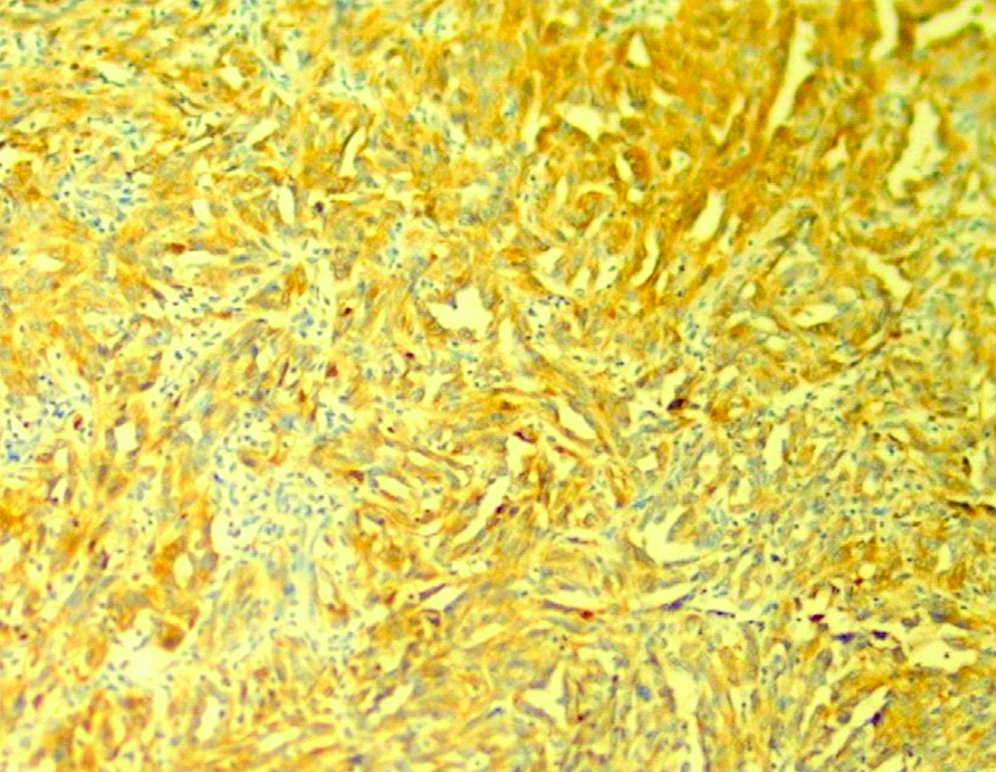
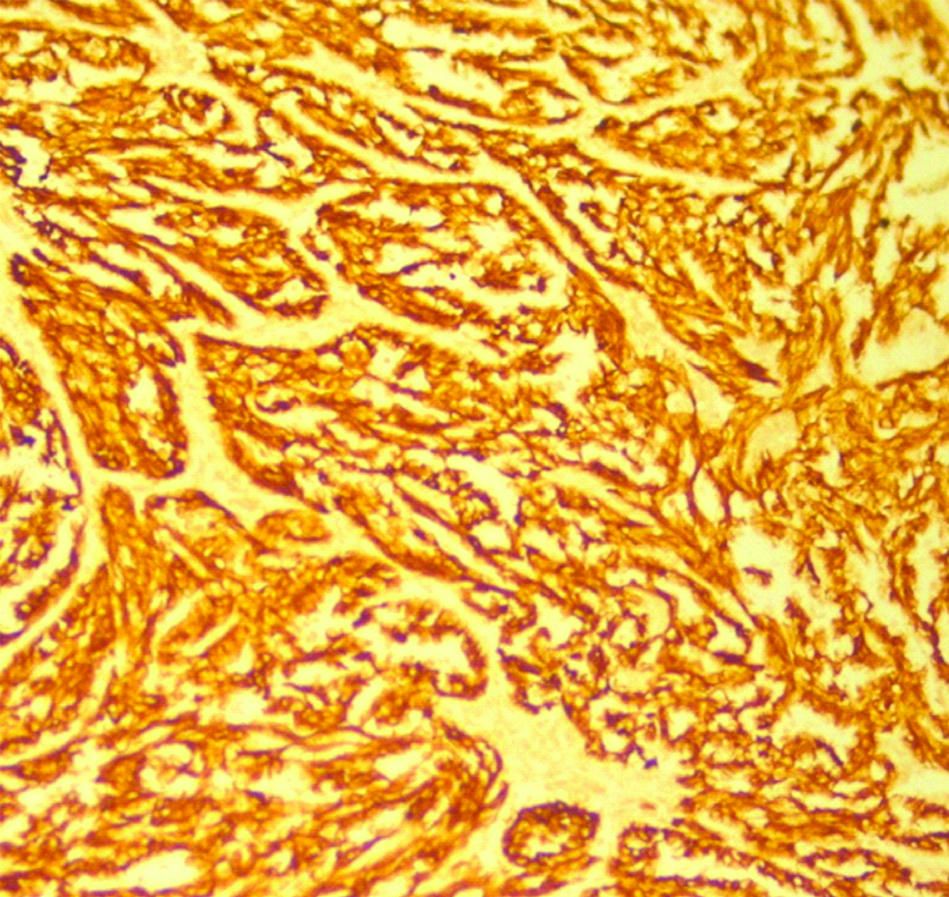
A 33-year-old female presented with complaint of abdominal pain with alternating constipation and diarrhoea for two months. There was increasing constipation from last one month. There was no history of malena or blood in stools. Her past history revealed that she was a known case of stage 1 Renal Cell Carcinoma (RCC) with left nephrectomy done 3 years back. There was no significant family history. It was papillary renal cell carcinoma, high grade with focal sarcomatoid component (10%) and extensive necrosis (50%). Size (10×8×6 cm) of the primary tumour, high grade, presence of sarcomatoid component and necrosis were the poor prognostic factors. All her hematological investigations were normal except for anaemia with haemoglobin 9 gm/dL. Abnormal biochemical investigation associated with poor prognosis were LDH-231 (Normal 135-225 IU/L), Alkaline phosphatase-161 (Normal– 4-130 IU/L). Serum calcium was normal. CT scan showed a nodular mass measuring 8×6×5 cm in descending colon [Table/Fig-1]. Clinical diagnosis of colonic tumour was made. Left hemicolectomy was performed. Right kidney was normal functioning. Gross examination showed a well defined, pedunculated mucosal polypoidal tumour measuring 8×5.5×4.8 cm along with a mesenteric nodule measuring 3.2×2.2×1.2 cm. On cut section, tumour was solid, firm, greyish white. Twenty-two lymphnodes were also removed largest measuring 1.7×1.2×0.8 cm [Table/Fig-2]. Histopathological examination shows a mucosal tumour in colon consisting of atypical spindle cells with abundant eosinophilic cytoplasm in sheets sarcomatous stromal appearance [Table/Fig-3,4]. Cells showed nuclear pleomorphism, apoptosis and atypical mitosis (1-2/ high power field) [Table/Fig-5]. There were no carcinoma element or necrosis present in the colonic tumour biopsy. Past history of RCC made it highly suspicious for a metachronus metastasis to colon. Immunostains were accordingly applied to differentiate between primary or secondary colonic tumour. Cells were strongly positive for pancytokeratin (pan CK), Epithelial Membrane Antigen (EMA), vimentin [Table/Fig-6,7] and negative for CK 7, CK 20, S100 and CDX2. HMB45, CD 117, CD34, desmin and smooth muscle actin were also negative. All the lymph nodes were negative for malignancy. A diagnosis of metastatic sarcomatoid RCC was made. Patient has been referred to higher centre in Riyadh for further treatment and is on Sunitinib+Gemcitabine therapy.
CT scan showing colonic mass (arrow), well defined, slightly enhancing related to descending colon. Absence of left kidney can also be appreciated.
Gross specimen showing a pedunculated mucosal polypoidal tumour in the colon.
A mucosal tumour in colon consisting of atypical spindle cells. (Haematoxylin and Eosin, 100x)
A mucosal tumour (thick arrow) is seen along with normal colonic glands (thin arrow). (Haematoxylin and Eosin, 200x)
Spindle cells having eosinophillic cytoplasm, round to oval pleomorphic nuclei with opened up chromatin and small eosinophillic nucleoli. (Haematoxylin and Eosin, 400x).
Positve PanCytokeratin (CK+) immunostaining. (100x).
Positve Vimentin immunostaining. (100x).
Discussion
RCC which originates from the lining of the proximal convoluted tubules within the renal cortex, is seen in 80 to 85 percent of all primary renal neoplasms. RCC is the 14th most common cause of cancer in the general population, accounting for 2%-3% of all new cancer cases detected per year worldwide. The estimated worldwide incidence of RCC is 15 cases per 100,000 populations but there is a higher incidence in males (annual incidence of 20.7 per 100,000 population) compared with females (annual incidence of 10.5 per 100,000 population) [1]. The commonest site of distant metastasis in 1451 autopsy cases with RCC was in the lungs (76%), followed by lymph nodes, bones, liver and brain. RCC can also metastasize to the adrenal glands, the contralateral kidney [2,3].
There is an increasing trend of kidney cancer over the last 2 decades in Saudi Arabia and it’s the third most common cancer of genitourinary tract after urinary bladder and prostate. However, it still has fewer patients with incidental kidney tumours as compared to developed countries. It accounts for 3.4% of all male cancers and 2.0% of all female cancers. In 2010, a total of 167 cases were diagnosed in males and 117 cases in females. Accurate incidence of RCC is not available and most of the studies are institutional [4,5].
Failed treatment with radiotherapy, multiple metastatic sites, sarcomatoid differentiation, elevated alkaline phosphatase, neutrophilia and thrombocytosis are poor prognostic factors [6].
Gastrointestinal (GI) metastasis from RCC is extremely rare and metastatic mass is indistinguishable from primary. Patients present with bowel complications like obstruction or intussusceptions as in the present case. There are very few case reports with mRCC to the GIT. Small intestine (ileum and duodenum) is the most frequently involved site. Metastasis to colon is extremely rare. RCC can metastasize to the GI tract through haematogenous or peritoneal seeding. GI metastatic lesions from RCC are mostly intraluminal polypoid masses as seen in the present case which may produce bleeding or features of obstruction [7,8].
Sarcomatoid RCC (sRCC) is not a separate subtype but can be seen in variable proportions with other histological subtypes of RCC and is metastatic in majority of the cases. However, sRCC metastatic to colon is extremely rare [9,10]. Any case of primary papillary carcinoma metastatic to colon as sarcomatoid RCC has not been found.
All the spindle cell lesions of GIT come in the differential diagnosis which includes leiomyosarcoma, epithelioid angiosarcoma, epithelioid Malignant Peripheral Nerve Sheath Tumour (MPNST), Gastrointestinal Stromal Tumour (GIST) and melanoma. The absence of S-100 and HMB-45 ruled out the possibilities of an epithelioid MPNST and melanoma. A leiomyosarcoma was ruled out in view of smooth muscle actin and desmin negativity. Lack of C-kit and CD34 ruled out the possibility of a GIST and an epithelioid angiosarcoma. Carcinosarcoma and Sarcomatoid carcinoma are very rare and aggressive tumours composed of carcinoma and mesenchymal cells make difficult and close differential diagnosis. Carcinosarcoma shows positivity for CK in carcinomatous ares and vimentin and SMA in sarcomatous areas. Sarcomatoid carcinoma is a very rare tumour of colon which usually presents with metastasis, shows CK and vimentin positivity in both carcinomatous and sarcomatous areas [11].
Metastatic RCC (mRCC) with sarcomatoid differentiation is an aggressive disease and represents as an aggressive subclone arising from the primary tumour. Sarcomatoid differentiation has shown frequent p53 mutations. Mutations in cyclin dependent kinase inhibitor (CDKN2A) and neurofibromoin 2 have also been reported [12]. Over-expression of Hypoxia Inducible Factor (HIF) pathway proteins like HIF1α, Vascular Endothelial Growth Factor (VEGF), mammalian Target Of Rifampicin (mTOR) proteins like phosphor S6, phosphor 4E BP1 and also programmed death ligand (PD-L1) which helps in evading the immune system may be responsible for aggressiveness of sRCC [13,14]. Epithelial to Mesenchymal Transition (EMT) in association with Transforming Growth Factor β (TGF β) and a relation between TGF β and m TOR complex required for cell migration and invasion has also been shown to be important in metastasis of sRCC [15].
Immunohistochemically, spindle cells are positive for cytokeratin and vimentin [16]. These tumours are generally positive for AE1/AE3, EMA and vimentin, which supports an epithelial origin, distinguishing them from renal sarcomas. Staining for actin, desmin, and S-100 are usually negative [17]. Clear cell RCC is associated with 80% of sRCCs with high frequency of sarcomatoid transformation also observed in patients with chromophobe RCCs [18].
Studies have reported favourable outcomes for surgically resected solitary metastatic disease. Multimodality approaches with combinations of chemotherapy (gemcitabine in combination with doxorubicin), stereotactic radiotherapy and targeted and non targeted agents are currently being evaluated for mRCC which include interferon α, Interleukin-2. Molecular targeting with VEGF inhibitors like sunitinib, sorafenib or bevacizumab and multi tyrosine kinase inhibitor axitinib which targets VEGF and mTOR inhibitor everolimus and adjuvant PD L1 inhibition is currently under evaluation [18-20].
Conclusion
Metastatic RCC with sarcomatoid differentiation is an aggressive disease. Colonic metastasis is extremely rare and all the spindle cell lesions of GIT come in the differential diagnosis. Considering distant metastasis, follow-up is an important part of the management of RCC. Further research and evaluation of multidisciplinary treatments especially targeted therapy should be pursued to improve the prognosis of sRCC.
[1]. Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, SEER Cancer Statistics Review 1975-2009 (Vintage 2009 Populations) 2012 [Google Scholar]
[2]. Saitoh H, Distant metastasis of renal adenocarcinomaCancer 1981 48:1487-91.10.1002/1097-0142(19810915)48:6<1487::AID-CNCR2820480635>3.0.CO;2-9 [Google Scholar] [CrossRef]
[3]. Capitanio U, Montorsi F, Renal cancerLancet 2016 387(10021):894-906.10.1016/S0140-6736(15)00046-X [Google Scholar] [CrossRef]
[4]. Alkhateeb SS, Alkhateeb JM, Alrashidi EA, Increasing trends in kidney cancer over the last 2 decades in Saudi ArabiaSaudi Med J 2015 36(6):698-703.10.15537/smj.2015.6.1084125987112 [Google Scholar] [CrossRef] [PubMed]
[5]. Cancer Incidence Report, Saudi Arabia, 2010. Available from: http://www.chs.gov.sa [Google Scholar]
[6]. Janzen NK, Kim HL, Figlin RA, Belldegrun AS, Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent diseaseUrol Clin North Am 2003 30:843-52.10.1016/S0094-0143(03)00056-9 [Google Scholar] [CrossRef]
[7]. Vo E, Palacio CH, Omino R, Link RE, Sada Y, Avo A, Solitary colon metastasis from renal cell carcinoma nine years after nephrectomy: A case reportIJS Case Reports 2016 27:55-58.10.1016/j.ijscr.2016.07.05327543725 [Google Scholar] [CrossRef] [PubMed]
[8]. Park HJ, Kim HJ, Park SH, Lee JS, Kim AY, Ha HK, Gastrointestinal involvement of recurrent renal cell carcinoma: CT findings and clinicopathologic featuresKorean J Radiol 2017 18(3)452-6010.3348/kjr.2017.18.3.45228458597 [Google Scholar] [CrossRef] [PubMed]
[9]. Zhao W, Yu Y, Chen Z, Huang X, Zhang Z, Colon metastasis of chromophobe renal cell carcinoma with sarcomatoid changeChin Med J 2012 125(18):3352-54. [Google Scholar]
[10]. Invernizzi R, Bencardino K, Porta C, Vercelli A, Viglio A, Manzoni M, Sigmoid colon metastasis from sarcomatoid renal cell carcinomaTumouri 2006 92:246-48.10.1177/030089160609200312 [Google Scholar] [CrossRef]
[11]. Lee JK, Ghosh P, Krinsky ML, Carethers JM, Evidence of colorectal sarcomatoid carcinoma arising from tubulovillous adenomaWorld J Gastroenterol 2008 14(27):4389-94.10.3748/wjg.14.438918666331 [Google Scholar] [CrossRef] [PubMed]
[12]. Mouallem NE, Smith SC, Paul AK, Sarcomatoid renal cell carcinoma: Biology and treatment advancesUrol Oncol- Semin ORI 2018 36:265-71.10.1016/j.urolonc.2017.12.01229306556 [Google Scholar] [CrossRef] [PubMed]
[13]. Tickoo SK, Alden D, Olgac S, Fine SW, Russo P, Kondagunta GV, Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinomaJ Urol 2007 177:1258-63.10.1016/j.juro.2006.11.10017382701 [Google Scholar] [CrossRef] [PubMed]
[14]. Joseph RW, Millis SZ, Carballido EM, Bryant D, Gatalica Z, Reddy S, PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiationCancer Immunol Res 2015 3:1303-07.10.1158/2326-6066.CIR-15-015026307625 [Google Scholar] [CrossRef] [PubMed]
[15]. Boström AK, Möller C, Nilsson E, Elfving P, Axelson H, Johansson ME, Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transitionHum Pathol 2012 43:708-19.10.1016/j.humpath.2011.06.01921992819 [Google Scholar] [CrossRef] [PubMed]
[16]. Kwak C, Park YH, Jeong CW, Jeong H, Lee SE, Moon KC, Sarcomatoid differentiation as a prognostic factor for immunotherapy in metastatic renal cell carcinomaJ Surg Oncol 2007 95:317-23.10.1002/jso.2066917066434 [Google Scholar] [CrossRef] [PubMed]
[17]. Kuroda N, Toi M, Hiroi M, Enzan H, Review of sarcomatoid renal cell carcinoma with focus on clinical and pathobiological aspectsHistol Histopathol 2003 18:551-55. [Google Scholar]
[18]. Shuch B, Bratslavsky G, Linehan WM, Srinivasan R, Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategiesThe Oncologist 2012 17:46-54.10.1634/theoncologist.2011-022722234634 [Google Scholar] [CrossRef] [PubMed]
[19]. Graves A, Hessamodini H, Wong G, Lim WH, Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalitiesImmunotargets Ther 2013 2:73-90.10.2147/ITT.S3142627471690 [Google Scholar] [CrossRef] [PubMed]
[20]. McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia studyJ Clin Oncol 2016 34:833-42.10.1200/JCO.2015.63.742126755520 [Google Scholar] [CrossRef] [PubMed]