Aneurysms are the most common cause of non-traumatic/SAH, accounting for approximately 80-90% of SAH [1,2], with a fatality rate of 40%-60% [3]. The gold standard for aneurysm detection is conventional catheter DSA, however, it is an invasive procedure with neurological complications in <1% of patients [2-7]. Also, it is a time-consuming procedure as compared to CTA. In the setting of emergency screening of SAH, multi-detector CTA, a non-invasive technique, plays an important role in accurately detecting aneurysms [3]. CTA is being increasingly used in the diagnostic triage of SAH. Review of literature shows a high sensitivity specificity, accuracy, PPV and NPV for 16-slice CTA (almost reaching up to 100%) for aneurysms >3 mm [2]. However, the smaller aneurysms of size less than 3 mm are detected by CTA with much lower sensitivity ranging between 74 to 84% [3]. Overall sensitivity and specificity for 16-slice CTA is 98.4% and 99.7%, respectively, on per patient basis, and 96.9% and 98%, respectively, on per aneurysm basis [4,5]. In our study-in addition to adding to the literature of accuracy of CTA, we have assessed the role of CTA in detecting and characterising the morphology of intracranial aneurysm in comparison with DSA and intraoperative findings also, wherever available.
Materials and Methods
This analytical study was conducted in the Department of Radiodiagnosis and Imaging, in collaboration with Department of Neurosurgery during March 2012 to March 2016. Majority of the patients had been referred for CTA from neurosurgery department in view of plain CT study showing SAH without any history of trauma, whereas few patients were incidentally detected/ suspected to have aneurysm based on plain-CT or MRI. So, all such patients over the age of 18 years, who underwent CTA with DSA for suspected intracranial aneurysm and whether or not intraoperative aneurysmal findings were available, were included in the study. Patients with contraindication to the use of contrast or radiation were not subjected to CT, like pregnant and lactating patient, severe renal failure, cardiac failure, multiple myeloma, previous allergic reaction to contrast media. Intranidal aneurysm within AVM, recurrence/residual aneurysms in postoperative or coiling cases were also excluded from the study.
CTA was performed on GE (General Electric, Milawaukee, United States), bright speed, 16-slice, multi-detector CT scanner. Informed consent was taken from all patients or their legally authorised representative. Ethics committee clearance was obtained at the institutional level. Informed consent for imaging was obtained from each patient.
CTA plain was obtained at a section thickness of 0.625 mm (tube voltage, 120 KV; tube current, 320 mA) and then 100 mL of non-ionic iodinated Iohexol contrast medium was administered via an 18-gauge needle, at a flow rate of 4 mL/sec using a pressure injector. Data was transferred to GE Advantage workstation version 4.2 and post-processing performed, MPR, 3D (Three Dimensional) reconstructions, 3D VR and MIP were performed by radiologist/trained technologist under supervision, using standard protocols.
DSA was performed on GE Advantax LC+ (single plane), omega 4, dedicated cardiac machine with MX 150 V3 only tube. The source of SAH and aneurysm were identified. Appropriate additional views were taken when necessary to delineate the neck of aneurysm, orientation and the origins of blood vessels overlapping the aneurysm. The data was transferred to workstation and measurements taken following catheter calibration.
Intraoperative images and on-table indocyanine angiography images were obtained using Leica neurosurgical operating microscope. Comparison of findings of CTA with DSA was performed. The aneurysms were assessed for morphological parameters, like size (dome, neck, height, dome to neck ratio, aspect ratio in saccular aneurysm and length and diameter in fusiform aneurysm), aneurysm angle, inflow angle; shape and lobulations; presence of tit on the aneurysm; location; presence of thrombosis and calcification; presence of vessels arising from the dome/neck of aneurysm; grade of SAH (modified CT Fischer score); aneurysm ruptured or not; and extent of vasospasm (grade of vasospasm, number and location of vasospastic segments).
Statistical Analysis
Statistical analysis was performed using SPSS version 17, to calculate the sensitivity, specificity, PPV and accuracy rate of both the diagnostic modalities in question. Fischer’s test was done and p-value of <0.05 was considered significant.
Results
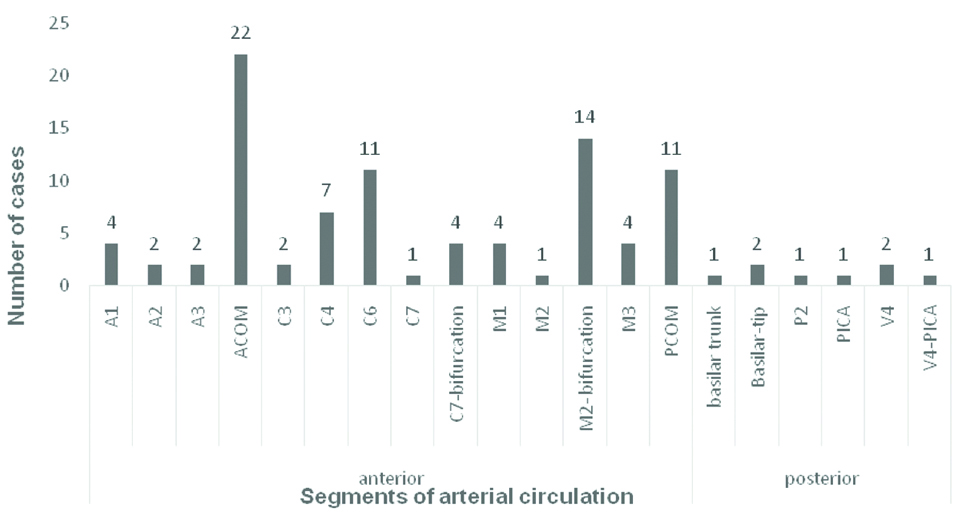
A total number of 71 patients were included in the study. There was a female predilection with male to female ratio of 1:1.53 with 60.5% (43 out 71 cases) females. Majority, that is; 25.3% (18 out of 71) cases were in the age group 60-69 years. This was followed by equal number of cases (22.5%) in the age groups 40-49 years and 50-59 years, followed by 16.9% and 8.4% in the age groups 30-39 years and 70-79 years, respectively. There were only 2 (2.8%) cases in the age group of 20-29 years and one (1.4%) case in the age group 80-89 years. There were in total of 97 aneurysms in the 71 patients and 54 aneurysms were operated. Eighty-nine (91.8%) of the aneurysms were in the anterior circulation and the rest eight (8.2%) were located in the posterior circulation [Table/Fig-1]. ACOM was the most common location of aneurysm, followed by M2 bifurcation, PCOM aneurysms respectively. In the ACOM region, 14 out of 22 aneurysms involved the ACOM and ACA junction, rest were exclusively in the ACOM. Fifty one (57.3%) of the saccular aneurysms were located at the bifurcation of arteries and 37 (41.6%) were in the side wall. There was only one (1.1%) aneurysm in a terminal location and this was a mycotic aneurysm. Among the 97 aneurysms, 89 (91.7%) were saccular, five (5.2%) were fusiform and three (3.1%) fusosaccular. Out of the 89 saccular aneurysms only 20 (22.5) were lobulated, of which 16 aneurysms were bilobed, rest three and one aneurysms were multi- and trilobed, respectively.
Location of the aneurysms.
With regards to size, majority, that is 36.1% of the aneurysm were of medium size (5-15 mm), followed by 30.9% small-sized (<5 mm) and 28.9% tiny aneurysms. There were three giant aneurysms (25-50 mm) and one large aneurysm (15-25 mm). The largest and smallest aneurysm measured 47 mm and 1.3 mm, respectively. Significant correlation of dome: neck and aspect ratios between CTA and DSA (p=0.0089) was noted.
Aneurysm morphology measurements as per CTA and DSA is depicted in [Table/Fig-2,3].
Aneurysm morphology measurements as per CTA.
| Morphological features | Mean (mm) | Std. Deviation | Minimum (mm) | Maximum (mm) | Percentiles |
|---|
| 25th | 50th (median) | 75th |
|---|
| Dome | 5.71 | 6.1 | 1.3 | 47.0 | 2.7 | 4.4 | 6.2 |
| Neck | 4.13 | 2.79 | 0.9 | 21.0 | 2.58 | 3.56 | 4.9 |
| Height | 6.08 | 5.11 | 1.2 | 34.0 | 3.1 | 4.82 | 6.93 |
| Dome: neck ratio | 1.44 | 0.94 | 0.55 | 6.29 | 0.94 | 1.17 | 1.53 |
| Aspect ratio | 1.63 | 1.12 | 0.3 | 5.83 | 0.93 | 1.3 | 2.15 |
| Vessel diameter | 2.7 | 1.22 | 0.5 | 8.0 | 1.88 | 2.35 | 3.1 |
| Size ratio-aneurysm and vessel | 2.63 | 2.28 | 0.23 | 14.0 | 1.22 | 2.13 | 3.18 |
| Aneurysm angle | 82.51 | 24.71 | 22.0 | 154.0 | 70.75 | 84.0 | 95.0 |
| Inflow angle | 110.75 | 41.67 | 8.7 | 172.0 | 88.0 | 121.0 | 141.0 |
| Length (fusiform) | 21.4 | 20.69 | 3.4 | 60.0 | 6.95 | 9.6 | 38.5 |
| Diameter (saccular) | 6.24 | 1.21 | 4.4 | 8.0 | 5.45 | 5.95 | 7.45 |
Aneurysm morphology measurements as per DSA.
| Morphological features | Mean (mm) | Std. Deviation | Minimum (mm) | Maximum (mm) | Percentiles |
|---|
| 25th | 50th (median) | 75th |
|---|
| Dome | 5.71 | 6.1 | 1.5 | 49.0 | 2.88 | 4.4 | 6.2 |
| Neck | 4.17 | 2.7 | 0.86 | 20.0 | 2.6 | 3.6 | 4.8 |
| Height | 6.2 | 5.2 | 1.4 | 34.0 | 3.1 | 4.86 | 7.4 |
| Dome: neck ratio | 1.4 | 0.81 | 0.56 | 4.88 | 0.91 | 1.15 | 1.62 |
| Aspect ratio | 1.63 | 1.08 | 0.35 | 5.95 | 0.9 | 1.29 | 2.09 |
| Vessel diameter | 2.63 | 1.26 | 0.4 | 8.0 | 1.8 | 2.30 | 3.1 |
| Size ratio-aneurysm to vessel | 2.82 | 2.53 | 0.27 | 16.0 | 1.33 | 2.15 | 3.4 |
| Length | 20.85 | 19.25 | 3.3 | 56.0 | 7.23 | 10.1 | 37.0 |
| Diameter | 6.0 | 1.12 | 4.3 | 7.6 | 5.05 | 6.15 | 6.9 |
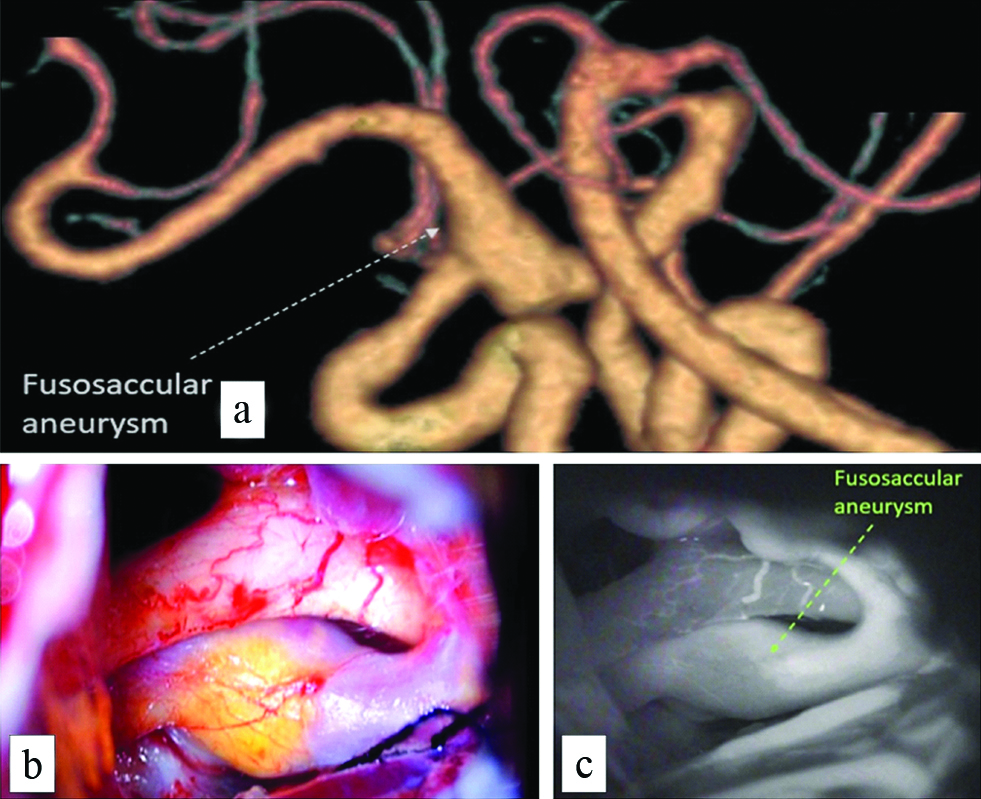
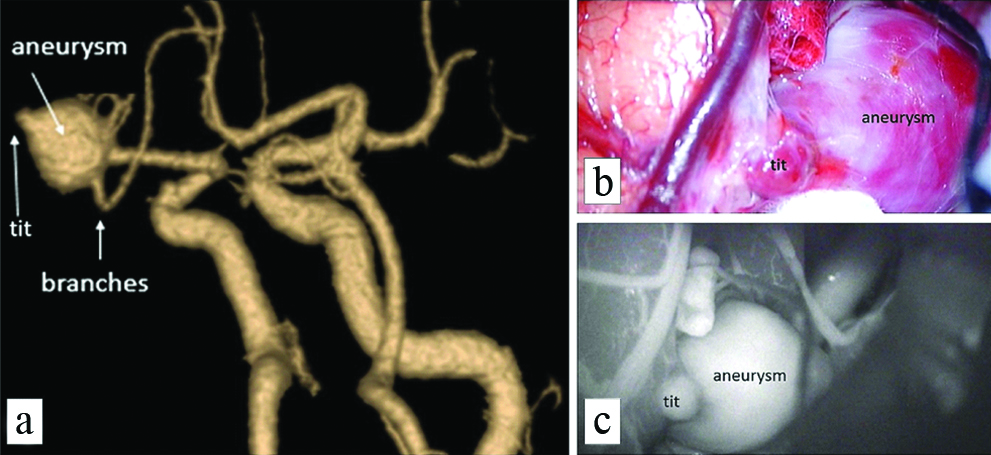
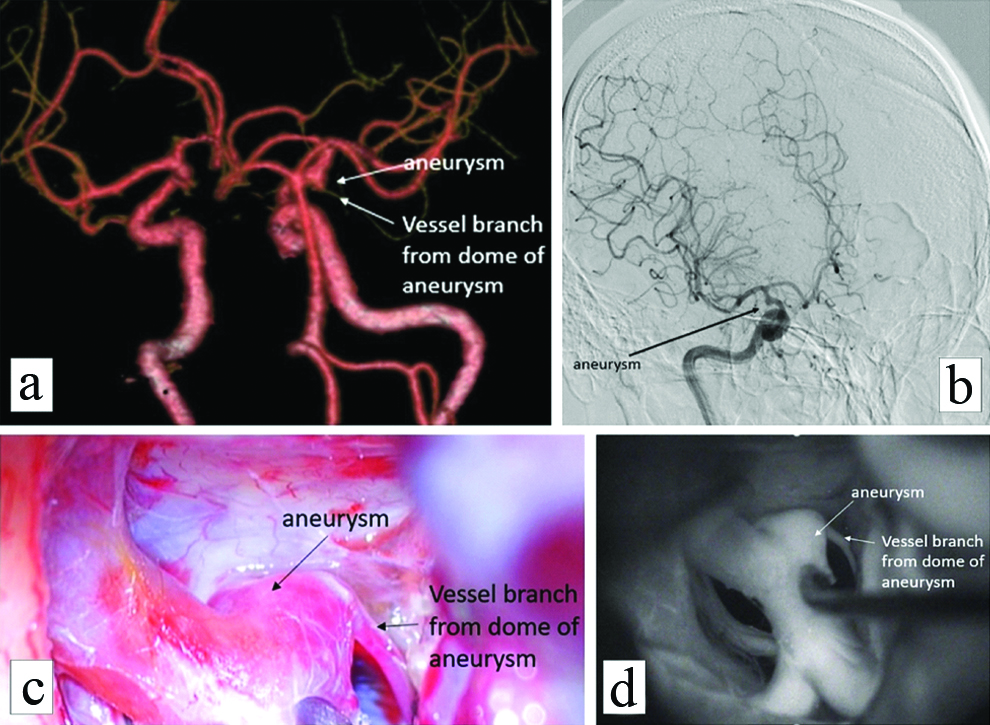
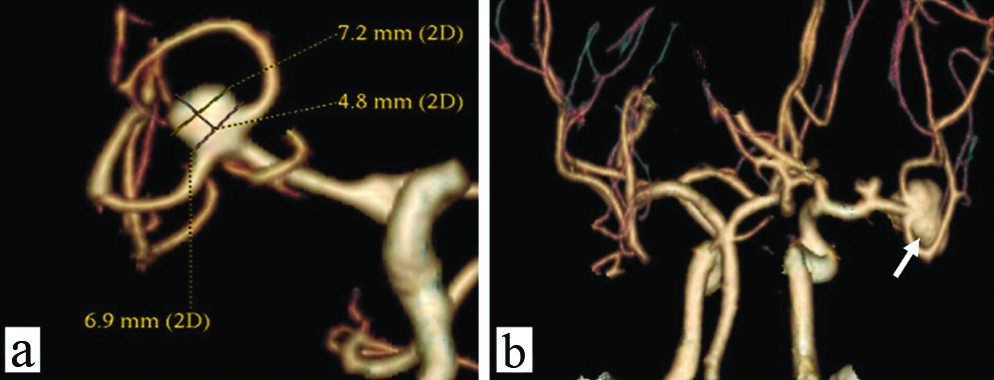
All the CTA studies were reformatted in volume rendered images in multiple views to look at the neck and other morphological parameters and compared to intraoperative images and intraoperative angiography wherever possible. [Table/Fig-4a] shows a fusosaccular aneurysm of the right ACA A1 segment seen on CTA. The same on intraoperative and indocyanine green angiography images in [Table/Fig-4b,c], respectively. A similar case of a saccular aneurysm of the MCA with tit of aneurysm which usually indicates the site of rupture of aneurysm is well visualised on CTA [Table/Fig-5a]; the same on intraoperative and indocyanine green angiography images [Table/Fig-5b,c]. Vessels in relation to the aneurysms were also studied as shown in [Table/Fig-6] displaying a saccular aneurysm of the MCA with a vessel arising from the dome of aneurysm well visualised on CTA [Table/Fig-6a] the same on DSA, intraoperative and indocyanine green angiography images [Table/Fig-6b-d] respectively. In this patient no clipping was performed instead muslin wrap was used. [Table/Fig-7] displays two other similar saccular aneurysms involving MCA at its bifurcation with superior and inferior divisions of M2 of MCA arising from the neck of the aneurysms well visualised on CTA.
Fusosaccular aneurysm of the right ACA A1 segment seen on CTA (a) dashed white arrow; the same on intraoperative and indocyanine green angiography images (b) and (c), respectively (dashed arrow).
A saccular aneurysm of the MCA with tit of aneurysm which usually indicates the site of rupture of aneurysm is well visualised on CTA (a, white arrows); the same on intraoperative and indocyanine green angiography images (b) and (c).
A saccular aneurysm of the MCA with a vessel arising from the dome of aneurysm is well visualised on CTA (a, white arrows) the same on DSA intraoperative and indocyanine green angiography images (b, black arrow), (c, black arrow) and (d, white arrow) respectively. In this patient no clipping was performed instead muslin wrap was used.
Two other similar saccular aneurysms involving MCA at its bifurcation with superior and inferior divisions of M2 of MCA arising from the neck of the aneurysms well visualised on CTA.
Two tiny aneurysms, located at M2 bifurcation and PCOM, of size less than 3 mm were missed by CTA which was picked up on DSA with a sensitivity of 92.86%, PPV of 96.3% and accuracy of 89.6%.
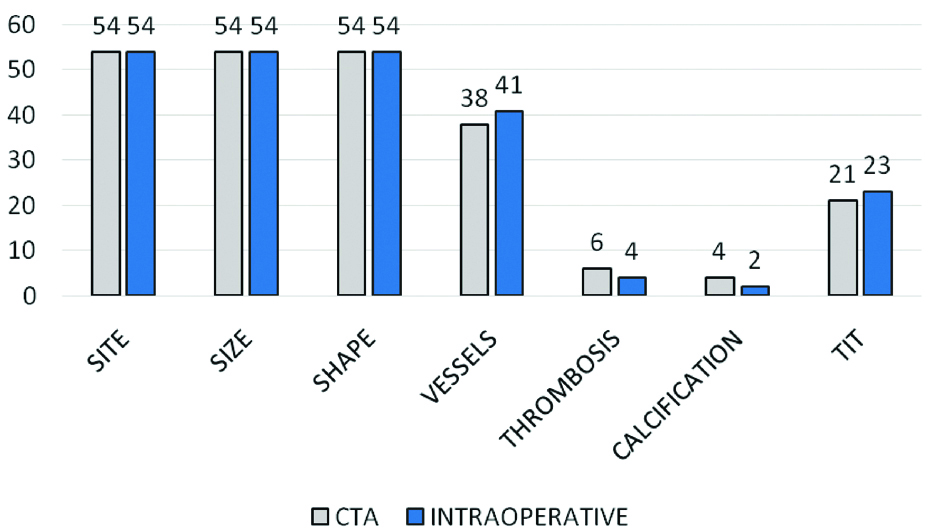
Thirty-four percent aneurysms were wide-necked. Forty-one aneurysms had vessel arising from the aneurysm. Majority were arising from the neck and correlated with the intraoperative findings in all the cases. Overall sensitivity of CTA irrespective of size of aneurysm as per our study was 97.9%, PPV of 97.9% and accuracy of 95.9%. A 54 of these aneurysms were operated and compared to the CTA and DSA findings [Table/Fig-8]. Thrombosis and calcification were better detected on CTA than DSA/intraoperative with a high statistical significance based on Fishers exact test with p-value of 0.0001 [Table/Fig-8]. CTA is also able to demonstrate vasospasm in the arterial segments as shown in [Table/Fig-9].
Table depicting CT angiography vs intraoperative findings.
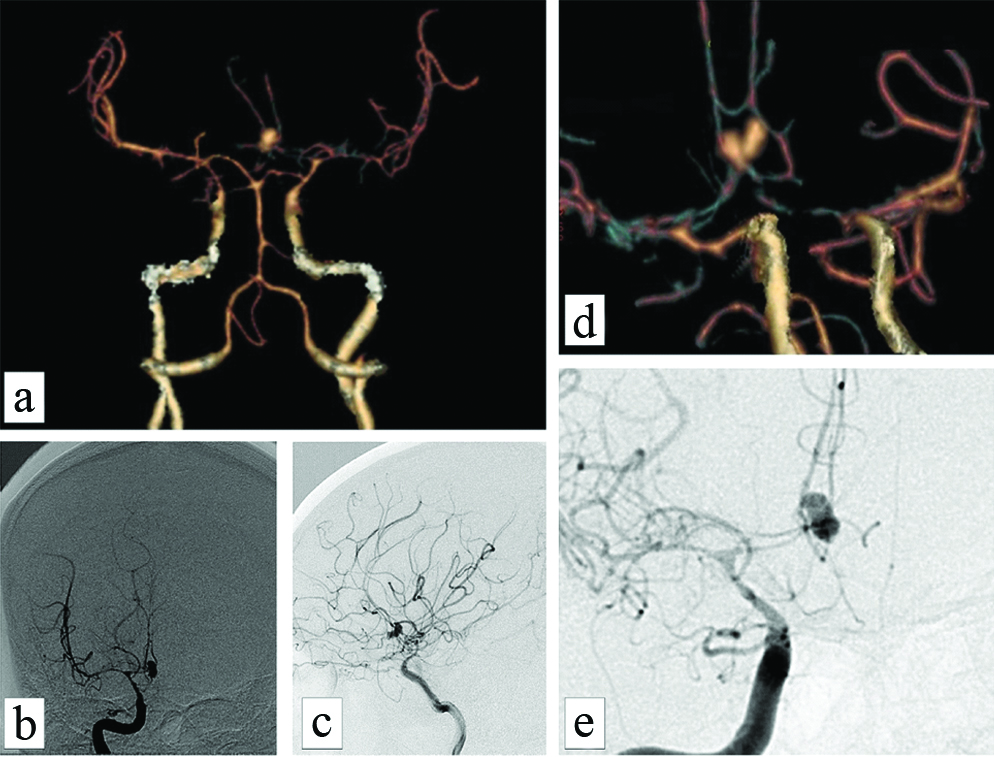
CTA of a 35-year-old female with grade IV subarachnoid haemorrhage (CT modified fischer score) following a bilobed aneurysm in the ACOM showing (a-d) significant grade-4 vasospasm predominantly involving bilateral A1 and A2 of ACA, C6 segments of bilateral ICA and M1 segments of bilateral MCA. same on DSA (b,c) also (e) zoomed images of the same showing the vasospastic segments.
Discussion
A literature seach regarding the role of CTA in SAH yielded high sensitivty and specificity in detecting aneurysms. Prestigiacomo CJ et al., conducted a prospective study in over a period of 36 months in 179 consecutive patients presenting with SAH who underwent CTA and DSA to assess the sensitivity and specificity and projected very high percentages [3]. Donmez H et al., compared 112 consecutive patients with suspected intracranial aneurysm using 16-row computed MDCTA with DSA. Each aneurysm based on sensitivity, specificity, and accuracy of MDCTA were 95.1%, 94.1%, and 95%, respectively. In aneurysms less than 3 mm size, sensitivity, specificity and accuracy of MDCTA were 86.1%, 94.1%, 88.6%, respectively [4]. In our study, the overall sensitivity of CTA achieved irrespective of size of aneurysm was 97.9%, PPV of 97.9% and accuracy of 95.9%. The sensitivity, PPV and accuracy of CTA for detecting aneurysm less than 3 mm was 92.86%, 96.3% and 89.66%, respectively. The sensitivity, PPV and accuracy of CTA for identifying aneurysm larger than 3 mm was 100%.
Menke J et al., did a meta-analysis to assess the accuracy of CTA for diagnosing intracranial aneurysms. Two independent reviewers selected 45 methodological studies comparing CTA with DSA and/or intraoperative findings. In a total of 3,643 patients, 77% of them had aneurysm. The pooled sensitivity and specificity based on per patient for CTA was 97.2% and 97.9% respectively for detecting aneurysms and based on per aneurysm was 95.0% and 96.2%, respectively [5]. Yoon DY et al., in a study of 85 patients obtained statistics with sensitivity, specificity, and accuracy of CTA were 92.5%, 93.3%, and 92.6%, respectively per-aneurysm. Pechlivanis I et al., studied the role of 3D CTA in deciding the management of SAH in patients older than 70 years. CTA and 2D-DSA were compared in 26 patients and there was good correlation in 96% of patients [7].
Chen W et al., did a study on 388 patients and concluded that 16 slice can provide sufficient information in guiding the surgical and endovascular therapy of aneurysm [8]. Chen W et al., performed a study and concluded that there was no significant difference between sensitivity of 16-slice CTA and conventional DSA (p>0.05) [9]. Westerlaan HE et al., performed a systematic review and meta-analysis and there was a pooled sensitivity of 98% (95% CI: 97%, 99%), and pooled specificity of 100% (95% CI: 97%, 100%) [10]. MacKinnon AD et al., performed a study on 200 cases and analysis showed that CTA had a high sensitivity and specificity [11].
Rai SV et al., performed a study to assess the effectiveness of CTA for detecting aneurysms in a 16 detector row CTA followed by three-dimensional reconstruction performed on patients with spontaneous SAH. In the study conducted on 50 patients with intracranial aneurysm, 48 cases were detected on CTA which was confirmed on the operating table. DSA was performed in doubtful cases and then diagnosed with aneurysm. The study showed that CTA has a sensitivity of 96.6% and specificity of 100%. Study concluded that the non-invasive, cost effective and easily performed CTA can replace the invasive DSA which involves a risk of neurological complication of 1%, costlier and needs high level of skill and thus it may be performed exclusively in case of ambiguity on CTA [12]. Dammert S et al., study showed that depending on the size of aneurysm the sensitivity of MSCT for small sized <4 mm aneurysm was 83.3%, medium sized 5-12 mm aneurysm was 90.6% and large size aneurysm was 100% [13].
CTA is a better modality to detect thrombosis and calcification correlating with previous studies and it is required to detect it especially if in the region of neck which may interfere with the clipping of aneurysm [14] and maximum intensity projections were better than volume rendering in detecting calcifications and thrombosis. Detecting arteries arising from the aneurysm is important because many a times the dome of the aneurysm is not exposed on table to prevent further haemorrhage and only the neck is visualised and clipped; so there is a chance of compromising a major vessel arising from the dome by inadvertently clipping neck of aneurysm and causing significant neurological deficit as in the case described in [Table/Fig-6]. Hence pre-op evaluation with CTA helps in detecting the vessels arising from aneurysm dome and neck. It is also important to assess vasospasm to prevent cerebral ischemia and infarction [15]. Using CTA we were able to detect vasospasm associated with 27 patients with a total of 54 segments of artery involvement as in the case described in [Table/Fig-9]. Multiple studies have used CTA to assess the morphology of the aneurysms as predictors of aneurysm rupture such as tits, high domes and small mean diameters [16,17].
Limitation
The limitations of this study were a low volume data and inability to acquire long term follow-up on the unruptured aneurysms.
Conclusion
Multidetector CTA with image reconstruction is the ideal first line imaging modality in non-traumatic SAH for detection of aneurysm with a high degree of accuracy. DSA can be used to diagnose tiny aneurysms less than 3 mm if ambiguity on CTA and if there is a high degree of suspicion. CTA helps to define the morphology, location and orientation of intracranial aneurysm. Morphological features like tit can be detected on the 3D volume rendering techniques. CTA is an ideal modality to detect thrombosis and calcification. Preoperative evaluation with CTA helps in detecting the vessels arising from aneurysm dome and neck. CTA helps to assess vasospasm.