Pregnancy is a physiological state of haemodynamic changes. The glomerular filtration rate and renal blood flow rise markedly during pregnancy [1]. These changes in flow rate and permeability of the glomerular basement membrane increase the urinary protein excretion substantially, leading to microalbuminuria [2].
In the general population, this urinary microalbumin estimation is a good screening test for early detection of renal disease and may be a marker for the presence of microvascular disease [3]. It is currently an indication of interventions, such as lowering of blood pressure even in the absence of hypertension or diabetes [4].
As albuminuria is one of the classical signs of pre-eclampsia, if microalbuminuria is present in a symptom-free female during pregnancy, it confirms that there are changes occurring in the renal function and the patient may develop pre-eclampsia eventually [5]. Thus, early pregnancy levels of microalbuminuria can be used as a predictor of pre-eclampsia [6]. All these physiological and pathological changes are associated with the dysfunction of autonomic nervous system. In pre-eclamptic patients, a higher excitability of sympathetic system has been noted [7]. In fact, in all hypertensive individuals, sympathetic hyperactivity is a generalised phenomenon, irrespective of the heterogeneous clinical aspects that accompany a high blood pressure state [8].
It has even been proposed that all the haemodynamic changes during pregnancy occur through autonomic control mechanisms [7]. Pre-eclampsia-related changes in the Autonomic Nervous System (ANS) based control of heart rate, including both sympathetic and parasympathetic divisions can be detected by a proper analysis of HRV. HRV is a measurement of the beat to beat fluctuation in the function of heart. It reflects an influence of ANS on heart rhythm [9].
Sympathetic activation is commonly seen in early stages of healthy pregnancy in spite of normally occurring reduced diastolic pressure [10]. Along with this finding, the sympathetic dominance is more prominent in pregnancy with PIH [11]. A Sudan based study has also found evidence for dominant cardiac sympathetic modulation in pregnant women with pre-eclampsia [12]. Also, a recent study has verified that autonomic nervous system imbalance, as well as insulin-resistance are impaired in PIH as compared to healthy pregnant women [13]. The clinical manifestations of PIH are said to be linked to placental ischaemia which releases cytokines into circulation leading to endothelial dysfunction [14]. Also, as earlier mentioned, microalbuminuria has been frequently reported in PIH.
Keeping in mind these information, several questions are raised. Is autonomic dysfunction in any way leading to placental ischaemia and microalbuminuria (as sympathetic activity causes vasoconstriction)? Is sympathetic dominance a feature of all pregnancies or it has any specific relation with prediction of PIH?
So, authors undertook to analyse HRV and urinary microalbumin excretion simultaneously in pregnant women. This would throw light on the pathophysiology of the occurrence of microalbuminuria with respect to possible sympathetic overactivity.
Materials and Methods
This is a hospital-based cross-sectional and prospective study conducted in the Department of Biochemistry, Subharti Medical College and Chhatrapati Sahuji Subharti Hospital, Meerut, Uttar Pradesh, India after obtaining ethical clearance from the Institutional ethical Committee. A total of 80 pregnant women (40 in 2nd trimester and 40 in 3rd trimester) attending antenatal clinic along with 40 matched controls were included in the study over a period of one and half year (June 2015 to Dec 2016). Sample size was determined keeping α-value at 0.05 and power of study 80%.
Source population: Both second and third trimester subjects were selected from the pregnant women attending the antenatal clinic of a tertiary level northern Indian hospital. Consecutively, controls were randomly selected from the vicinity after age matching (18-40 years; mean±SD- 25.79±4.15).
Subjects with acute anxiety, severe stress, previous history of eclampsia, uncontrolled hypertension, diabetes mellitus, gestational hypertension, gestational Diabetes Mellitus or any other endocrine/autonomic disorders and patients suffering from any acute or chronic illness like renal failure, cardiovascular disease, and hepatitis were excluded from the study.
As per the guidelines of National Kidney Foundation, spot morning Urine measurement of Albumin to Creatinine Ratio (UACR) was measured for proteinuria [15]. Spot midstream urine sample was collected in a sterile container and stored at –20°C refrigerator with proper labels and seal (Parafilm) till further test. Urinary Microalbumin was estimated using kit from VITROS 5.1 FS Chemistry system manufactured by Johnson and Johnson. The basic test principle utilises a turbidimetric inhibition based immune-assay technology. Urinary creatinine estimation was done after 21 times dilution with reagent-grade water as recommended on Vitros 5.1 FS chemistry autoanalyser. All samples were stored for not more than a month before analysis.
Urinary Albumin Creatinine Ratio (UACR) was calculated using the following formula [16]:
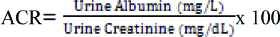
To find out the status of cardiac autonomic nervous system, heart rate variability was recorded in the Physiology Department using RMS POLYRITE-D-Analog/PC based Polygraph/Physiograph. Experiment was done in a quiet room during which subjects were made to lie down in a supine position. For recording of short-term HRV, lead II ECG recording was done (25 mm/s and voltage at 10 mm/mV) for 550 seconds to obtain HRV. The data recording was subjected to time domain {(mean respiratory rate in seconds, mean heart rate in beats per minute, standard deviations of the averages of Normal to Normal (N-N) intervals (SDNN), root mean square of differences of successive N-N intervals) (RMSSD) and frequency domain analysis {Total Power (TP) in ms2, Low Frequency (LF) component (0.04-0.15 Hz) in normalised units (nu) and High Frequency (HF) component (0.15-0.4 Hz) in normalised units (nu)}. LF-HF ratio was done using the HRV analysis software RMS Polyrite D version 3.0.7 (Chandigarh, India)}. The two main components of HRV spectrum are HF component (representing synchronicity) and LF component (representing sympathetic activity). In healthy subjects, LF:HF ratio represents a measure of sympathovagal balance. A higher LF:HF ratio suggests sympathetic dominance (European Task Force, March 1996 guidelines) [17].
Statistical Analysis
The Statistical software Graphpad Prizm (version 7.04) was used for the analysis of the data using various statistical tests. Student’s t-test and ANOVA were used for group comparison. Association between various groups was done using Pearson’s correlation coefficient. All data are reported as mean±standard deviation/standard error of mean (mean±SD/SEM). Significance was considered when p-value was calculated to be less than 0.05.
Results
The cases comprised of 80 pregnant women (40 in 2nd trimester and 40 in 3rd trimester of pregnancy). Forty age-matched non-pregnant women were also recruited for the study. [Table/Fig-1] shows the comparison of physiological parameters. The average blood pressure in the cases was 113.25±10.57 (systolic) and 71.19±8.19 (diastolic) which is comparable to the controls. So, all the cases were normotensive during the study.
Comparison of clinical variables in Cases vs Controls.
| Variables | Cases (Mean±SD; Range) n=80 | Controls (Mean±SD; Range) n=40 | p-value |
|---|
| Age (years) | 25.44±3.48 (19-36) | 26.50±5.23 (21-38) | 0.188 |
| Height (cm) | 156.24±4.83 (145-170) | 154.65±5.45 (145-175) | 0.107 |
| Weight (kg) | 62.76±10.43 (38-98) | 54.75±7.42 (38-67) | <0.001** |
| SBP (mmHg) | 113.25±10.57 (90-140) | 111.00±11.36 (90-130) | 0.286 |
| DBP (mmHg) | 71.19±8.19 (60-100) | 68.60±4.30 (60-76) | 0.064 |
SD: Standard deviation; DBP: Diastolic blood pressure; SBP: Systolic blood pressure
p-value measured by student’s t-test
The Microalbumin levels checked in the second trimester of pregnancy was 70.29±51.85 and in the third trimester were 58.71±36.15, whereas in the control group it was 17.65±14.19. This showed that the levels of Microalbumin were found to be high in the pregnant group and in them was higher in the 2nd trimester as compared to the 3rd trimester (p<0.001). The urinary spot ACR showed values of 84.48±52.61 in 2nd trimester, 72.35±50.29 in 3rd trimester, and only 17.59±6.19 in the non-pregnant control group. This again implies that ACR is raised in pregnancy (p<0.001) [Table/Fig-2].
Comparison of clinical parameters in three groups studied.
| Variables | 2nd trimester of pregnancy(Mean±SEM; Range)n=40 | 3rd trimester of pregnancy(Mean±SEM; Range)n=40 | Control(Mean±SEM; Range)n=40 | p-value(2nd trimeter vs control) | p-value(3rd trimeter vs control) |
|---|
| Microalbumin (mg/L) | 70.29±8.2 (6-190) | 58.71±5.7 (6-153.8) | 17.65±2.2 (6-58.3) | <0.001** | <0.001** |
| Albumin Creatinine ratio | 84.48±8.3 (23.8-261.55) | 72.35±8.0 (7.45-236.33) | 17.59±1.0 (7.9-44.4) | <0.001** | <0.001** |
**very significant, SEM: Standard error of mean; p-value calculated using ANOVA
The next parameter studied was heart rate variability. This is further divided into time domain analysis and frequency domain analysis. As authors’ analysis involved short-time recording (i.e., 10 minutes), authors emphasised more on frequency domain analysis as per European Task Force March 1996 guidelines [16]. In the Frequency domain, parameters studied were TP, LF, HF, LF/HF ratio.
The TP was 710.76±10.45 in pregnant and 1073.87±14.90 in non-pregnant. The pregnant group was further divided into 2nd trimester which showed value of 770.62±12.24 and 3rd trimester, which showed value of 652.80±8.29. This analysis suggested that TP was higher in non-pregnant females than pregnant group. The LF was 63.13±11.57 in pregnant and 46.38±14.86 in the non-pregnant. The pregnant group showed LF value of 62.33±12.71 in the 2nd trimester and 63.92±10.41 in the 3rd trimester. This showed that values of LF were higher in the pregnant group. The HF was 35.09±12.37 in pregnant and 53.13±14.50 in non-pregnant group. The pregnant group showed HF value of 37.03±12.94 in 2nd trimester and 33.15±11.60 in the third trimester. This showed that HF values were higher in non-pregnant group. The LF:HF ratio was 2.09±0.91 in pregnant and 1.04±0.65 in non-pregnant group [Table/Fig-3].
Comparison of study variables in three groups studied-Frequency domain.
| Variables | 2nd trimester of pregnancy(Mean±SEM; Range) | 3rd trimester of pregnancy(Mean±SEM; Range) | Control (Mean±SEM;Range) | p-value (2nd trimester vscontrol) | p-value (3rd trimester vscontrol) |
|---|
| Total Power (TP) {millisecond square} (Square root) | 770.62±1.93(136.75-4645) | 652.80±1.31(117.8-1863) | 1073.87±2.36(133.3-4645) | 0.12 | 0.027* |
| Low Frequency (LF) {Normalised unit} | 62.33±12.71(34.44-79.71) | 63.92±10.41(39.91-79.88) | 46.38±14.86(13.14-75.38) | <0.001** | <0.001** |
| High Frequency (HF) {Normalised unit} | 37.03±12.94(20.29-65.55) | 33.15±11.60(15.63-60.06) | 53.13±14.50(28.61-86.85) | <0.001** | <0.001** |
| Ratio (LF:HF) | 2.01±0.93(0.53-3.9) | 2.16±0.90(0.66-3.9) | 1.04±0.65(0.15-2.49) | <0.001** | <0.001** |
| 2.09±0.91 | | | |
*significant; **very significant; SEM: Standard error of mean; LF:HF: Low frequency: High frequency ratio as in frequency domain analysis in HRV analysis software RMS Polyrite D version 3.0.7 (Chandigarh, India)
p-value calculated using ANOVA
This analysis shows that mean value of HF was significantly reduced in pregnancy and mean values of LF:HF and LF were significantly increased as compared to the non-pregnant females. This shows the sympathetic dominance is more during pregnancy as compared to non pregnant.
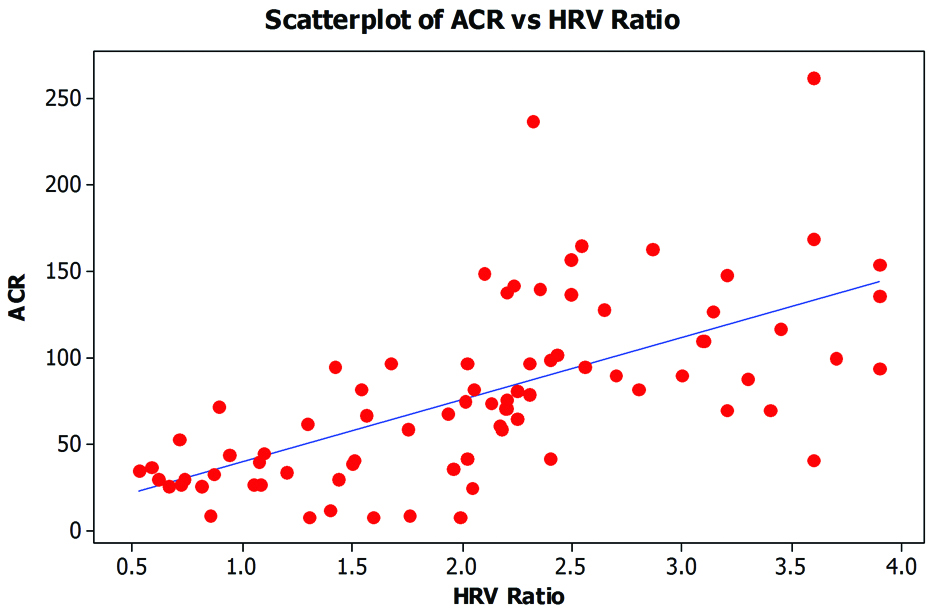
Correlation study done between heart rate variability ratio and albumin-creatinine ratio showed that in the 2nd trimester, the Pearson’s correlation value was 0.742, which suggests a very large correlation between the two, with a p-value of <0.001, which was strongly significant. The same was also done for the 3rd trimester which showed Pearson’s correlation value was 0.562, which suggests a large correlation between the two, with a p-value of <0.001, which was strongly significant. Overall, the urinary albumin-creatinine ratio increases as the HRV ratio rises, making it a statistically significant finding [Table/Fig-4]. Present study shows that there is a positive correlation between HRV and ACR which suggests that with the rising levels of LF/HF, the microalbuminuria also increased in all pregnant women (in both 2nd and 3rd trimester).
This is the scatter plot of Urinary Albumin Creatinine Ratio (UACR) and Heart Rate Variability (HRV) of all subjects. X axis denotes UACR in mg/g and y axis is representing the HRV ratio between low frequency and high frequency measurements on RMS POLYRITE-D-Analog/PC based Polygraph/Physiograph. r=0.694, p=<0.0001.
In the pregnant group, 62 out of 80 (77.5%) women developed microalbuminuria [Table/Fig-5]. Follow-up on delivery was possible in 50 of these 80 cases, of which 33 (41%) of them had developed PIH and it’s complications. This is a significant finding (OR=42.02, p-value <0.05). To keep value at 0.05 and power of study 80%, the minimal sample size required is 15 in case group and 7 in control group with the current UACR and LF:HF ratio values. So, with 30 patients lost to follow-up, 50 patients and 40 controls formed the required sample.
Table showing follow up data of cases.
| Total cases | Microalbuminuria present | Follow-up done in cases | PIH present | PIH absent |
|---|
| 80 | 62 | 50 | 33 | 17 |
Preterm labour was seen in 20 cases, while Preterm Premature Rupture of Membranes (PPROM) was seen in six. Offsprings of 23 of these cases had IUGR. In fact, All the 33 out of 50 followed up cases (66%) who had developed preeclampsia had HRV ratio of more than 1.5.
Discussion
Nearly one-tenth of all maternal deaths are associated with hypertensive disorders of pregnancy in countries like Asia and Africa, [18]. In India, the incidence of pre-eclampsia in pregnancy is 8-10% [19]. Normal pregnancy is associated with an increase in sympathetic regulation and decrease in parasympathetic influence over heart rate. These changes are increased in PIH [7].
As microalbuminuria in early pregnancy is known to be a predictor of the onset of pre-eclampsia, authors need to investigate the possible mechanism for the causation of this particular condition [20]. All the cases studied were normotensive during initial workup. However, on urine investigation, microalbumin levels and thus UACR ratio was significantly higher in the group of pregnant women as compared to the control group. This is in concordance with a study in Southern India where microalbuminuria was found to be a predictor of PIH [21] and another study in Paris where it was found that microalbuminuria developing during the course of pregnancy should be considered an important risk factor for development of PIH [20]. In the present study, the control group had no evidence of microalbuminuria. All these 50 cases which were followed-up had microalbuminuria. Sympathetic dominance was seen in 33 out of these 50 followed-up cases who later on developed pregnancy-induced hypertension (HRV ratio >1.5). The rest 17 cases who didn’t have sympathetic dominance during early visit did not develop PIH later on.
Spot UACR is a reasonable method for identification of proteinuria (>0.3 g/24 hour) during pregnancy as per the Society of Obstetric Medicine in Australia and New Zealand and also Canadian Society for Obstetrics and Gynecology [22]. Thus present study was done using UACR (spot) as per above guidelines [15].
Studies suggest that microalbuminuria occurs due to diffuse endothelial injury and ongoing kidney damage [23,24]. Due to glomerular endotheliosis, there may be increased leakiness of the glomerular filtration membrane and also there is reduced renal blood flow and tubular function. All these factors lead to increased filtration and reduced reabsorption of smaller plasma protein such as albumin leading to microalbuminuria initially. If the damage continues to progress, it may end up in gross proteinuria. Therefore, presence of microalbuminuria should be taken as a signal for impending serious renal damage. Microalbuminuria can be found long before the manifestations such as oedema, hypertension, renal failure etc., sets in [23].
In a recent Indian study, it was concluded that those fetal complications were more associated with babies of pregnant women with microalbuminuria. Maternal complications were also more with microalbuminuria group [24]. In another Indian study, cardiac modulation was studied in pregnancy by non-invasive heart rate variability measurement. They showed that sympathetic dominance is more during 3rd trimester of pregnancy as compared to first trimester of gestation [25], which is nearly similar to the present results. Even the present study showed that pregnant women with microalbuminuria had more complications in term of Pre-eclampsia, preterm labour, IUGR and PPROM. Thirty-three of present cases (66%) with LF:HF ratio of more than 1.5 had developed pre-eclampsia.
In another study of heart rate variability based in Aligarh, India, the LF/HF ratio was 6.8±4.52 and in the control group was (3.168±2.06) [26], whereas, in the present study group, the ratio was 2.09±0.91 and in the controls it was 1.04±0.65 with a p-value of <0.001, which was statistically strongly significant.
A Taiwanese study found that normal pregnant women had a lower HF value but had a higher LF/HF value as compared with the non-pregnant group. Likewise, The pre-eclamptic group had lower HF but higher LF/HF as compared to normal pregnant group. These results suggested that normal pregnancy is associated with a higher sympathetic regulation and diminished parasympathetic influence of heart rate, and such alterations are increased pre-eclamptic pregnancy, which is similar to the present study [27].
Limitation
The main limitation of the present study is the small sample size and also the fact that 37.5% cases were lost to follow-up which limits the prospective nature of the study.
Estimating placental pathological contributions in the form of proinflammatory markers like various cytokines could have been done to better explain the pathophysiology of pre-eclampsia, the autonomic function could be explored biochemically by assaying catecholamine levels, but these could not be attempted owing to lack of funds. However, the fact that HRV measurement alone has been correlated poses as strength of the study as it is noninvasive procedure and the futuristic pregnancy screening could be better accepted by the patients.
The cases on identification of microalbuminuria and sympathetic dominance were not intervened with any extra treatment/advice which leaves a scope for possible randomised control trials for better analysis. There remains a regional bias in this study as all cases and controls are based in a North Indian suburb.
So, for declaring a neurogenic role in microalbuminuria and subsequent development of pre-eclampsia, similar studies should be attempted elsewhere.
Future Scope
As this subclinical yet easily screenable condition predicts the development of pre-eclampsia/eclampsia in later pregnancy, all the methods targeting generalised stress reduction could be advised to all pregnant women during their first visit. Regular follow-up in pregnancy may also include HRV measurement and urinary microalbumin levels so that occurrence of pre-eclampsia can be prevented by early detection of its signs.
Heart Rate Variability (HRV) monitoring throughout antenatal visits can direct the need for UACR testing in the laboratory in a normotensive pregnant woman.
Conclusion
In the present study, there is a significant positive correlation between Heart Rate Variability (HRV) and microalbuminuria in pregnancy. This is a new finding in literature so far. This indicates the role of sympathetic hyperactivity in causing pregnancy-induced microalbuminuria which is known to prelude pregnancy-induced hypertension/pre-eclampsia. The fact that stress induced sympathetic overactivity is probably leading to microalbuminuria which is a common feature in the patients who ultimately developed pre-eclampsia, needs to be explored further. Microalbuminuria could be a coexistent finding in pre-eclampsia or it could be directly involved in the pathophysiology, this has to be elucidated in detail, as, this study as well as several previous studies, have noticed microalbuminuria in pregnancy, more so in Pregnancy induced hypertension (PIH).
This study however, shows that there is a neurogenic role for the causation of microalbuminuria in pregnancy which could further lead to preeclampsia.
SD: Standard deviation; DBP: Diastolic blood pressure; SBP: Systolic blood pressure
p-value measured by student’s t-test
**very significant, SEM: Standard error of mean; p-value calculated using ANOVA
*significant; **very significant; SEM: Standard error of mean; LF:HF: Low frequency: High frequency ratio as in frequency domain analysis in HRV analysis software RMS Polyrite D version 3.0.7 (Chandigarh, India)
p-value calculated using ANOVA