Prevalence of Class I and II Integrons among MDR Enterobacter cloacae Isolates Obtained from Clinical Samples of Children in Kermanshah, Iran
Seyed Hamidreza Mortazavi1, Faizullah Mansouri2, Mohsen Azizi3, Amirhooshang Alvandi4, Ali Karbasfrushan5, Nahid Madadi-Goli6, Sepideh Fereshteh7, Kamal Ahmadi8
1 Assistant Professor, Department of Paediatrics, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2 Associate Professor, Department of Infectious Disease, Kermanshah University of Medical Sciences, Kermanshah, Iran.
3 Research Assistant, Department of Microbiology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4 Associate Professor, Department of Microbiology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
5 Assistant Professor, Department of Anesthesiology, Critical Care and Pain Management, Kermanshah University of Medical Sciences, Kermanshah, Iran.
6 PhD Student of Medical Bacteriology, Department of Microbiology, Pasteur Institute of Iran, Tehran, Iran.
7 PhD Student of Medical Bacteriology, Department of Microbiology, Pasteur Institute of Iran, Tehran, Iran.
8 Research Assistant, Department of Microbiology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kamal Ahmadi, Research Assistant, Department of Microbiology, Faculty of Medicine, Kermanshah University of Medical Sciences, Shirudi blvd, Parastar blvd, Postal code: 6714869914, Kermanshah, Iran.
E-mail: k.ahmadi@kums.ac.ir; kamal.ahmadi55@yahoo.com
Introduction
Enterobacter species are among one of the key causes of hospital infections. The transfer of drug resistance genes through the integrons promotes the development of antibiotic resistance and the emergence of Multidrug Resistant (MDR) strains.
Aim
The aim of this study was to determine the prevalence of class I and II Integrons among MDR Enterobacter cloacae isolates obtained from clinical samples of children in Kermanshah, Iran.
Materials and Methods
This descriptive cross-sectional study was done during 11 month period from October 2016 to September 2017, 72 isolates of E.cloacae were collected from children under 15 years of age in Mohammad Kermanshahi Hospital in Kermanshah, Iran. After confirmation of the isolates with biochemical specific tests, their antibiotic susceptibility with disk diffusion was examined. Then the frequency of Class I and II integrons was determined by using their specific primers and by PCR method. Data was analysed by SPSS software version 20 and p-values less than 0.05 were considered statistically significant.
Results
The highest and lowest frequency of isolates were in blood samples 29 (40.3%) and CSF 2 (2.8%) respectively. From the 72 isolates of E.cloacae, 54 isolates (75%) were MDR. The highest antibiotic resistance was observed against Ampicillin-clavulanic acid (94.4%) and Cefalexin (69.4%), whereas the lowest antibiotic resistance was to Imipenem (9.7%) and Colistin (6.9%). Genotypically, the frequency of class I integron was 42 (58.3%), but none of the isolates had class II integron.
Conclusion
The results of this study demonstrates that E.cloacae isolated from children, in addition to the high frequency of MDR isolates, the prevalence of isolates with integron is expanding. Therefore, keeping with the role of integrons in resistance to different antibiotics, it is necessary to pay greater attention to identify them.
Antibiotic resistance, Drug resistance, Enterobacteriaceae
Introduction
Enterobacter is a gram-negative bacteria of the family Enterobacteriaceae and one of the causative agent of hospital infections. Various species of Enterobacter can cause disease in human, indeed E.cloacae and E.aerogenes are the main pathogens that are responsible for nosocomial infection [1,2]. This is because E.cloacae has many virulence factors such as the ability to secrete cytotoxin, enterotoxin, haemolysin and biofilm formation. The most common nosocomial infections caused by this organism are urinary tract infection, pneumonia, surgical wound, soft tissue, skin infection and bacteraemia [3,4]. Hospitalized children are more sensitive to infection by various pathogens such as Enterobacter and the prevalence of infection by this bacterium as a significant pathogen is increasing in neonatal intensive care unit [5,6]. Treatment of this bacterial infection by various broad- spectrum Cephalosporins have resulted in the development of resistance against them. There are many reports of increasing MDR E.cloacae isolates [7,8]. Recently, the treatment of bacterial infection has been difficult due to misuse of antibiotics and spreading of antibiotic resistance isolates [9]. Among the effective factors in the development and acquisition of drug resistance genes by bacterial isolates, mobile genetic elements, including plasmids, integrons and transposons have been considered significant. In the meanwhile, integrons are so important because they have a specific recombination system that causes insertion and expression of various genetic cassettes. Horizontal transformation of integrons is known as the most effective way of spreading antibiotic resistance genes, which results in MDR [10]. Integrons are embedded in plasmids, chromosomes, transposons; cause transmission and extension of resistant gene in the genetic cassette. So far, different classes of integrons have been identified based on their variation on integrase. The most prevalent integron, is class I integron and has sull gene which is mostly found in gram positive and gram negative bacteria isolated from the clinical samples. Class II integron is embedded in Tn-7 transposon and Class III contain metalobetalactamase gene. These mobile elements play a role in the transmission of a large number of drug resistance gene and as a result acquires resistance to various antibiotics including beta-lactam antibiotics, Macrolides, Aminoglycosides and others [11-13]. No studies have been done on the prevalence of Enterobacter infection in children and their antibiotic resistance pattern in Kermanshah, so far, the aim of this study was to determine the prevalence of class I and II integrons among MDR E.cloacae isolates obtained from clinical samples of children in Kermanshah, Iran.
Materials and Methods
This descriptive cross-sectional study was done during 11 month period from October 2016 to September 2017, on 72 isolates of E.cloacae isolated from blood, urine, sputum, Bronchoalveolar Lavage (BAL), wound and CSF of children under 15 years of age in Mohammad Kermanshahi hospital in Kermanshah, Iran. In this study, informed consent was obtained from the parents of the children. Only the clinical specimens of children hospitalized at the age less than 15 years infected with E.cloacae were included. Also, other Enterobacter species and samples from patients over the age of 15 years were excluded. Samples were collected and transferred to laboratory and were cultured on a special media MacConkey agar and EMB agar in a sterile condition. Then, for identification of Enterobacter, specific tests including culture in IMVIC and TSI were used. Finally, 72 isolates of E.cloacae were confirmed and investigated. Using the Kirby-Bauer disk diffusion test and 15 antibiotic disks (MAST, U.K.) namely Ampicillin-clavulanic acid, Cefalexin, Amikacin, Gentamycin, Colistin, Nalidixic acid, Ceftazidime, Cefixime, Cefotaxime, Ciprofloxacillin, Norfloxacin, Cotrimoxazole, Imipenem, Nitrofurantoin and Aztreonam was used to determine the antibiotic resistance pattern of the isolates. Firstly, a colony of bacteria was inoculated in Muller Hinton broth and after two hours and comparing it with McFarland standards, this suspension was cultured on Muller Hinton agar for antibiogram test. Next small disks containing different antibiotics, was placed in different zones of the culture on an agar plate. After 24 hours of incubation at 37°C, the results were compared with CLSI standard tables [14]. A standard E.coli strain, ATCC 25922 was used as a quality control for antibiogram test. According to the definition, isolates that are resistance to three or more major classes of antibiotic was considered MDR. To extract the chromosomal DNA of isolates boiling method was used. To do this, the pure colonies were dissolved in 0.5 mL of sterile distilled water and after five minutes of boiling and cooling, were centrifuged for one minute at 7000 g. After centrifuge, the tube was taken out and the supernatant containing bacterial DNA transfered into a new sterile micro-tube for PCR reaction. Then PCR reaction was conducted to identify class I and II integrons by specific primers and total volume 25 microliter including 12.5 microliter master mix, one microliter of each primer, two microliter DNA and sterile distilled water. The temperature cycle of the PCR reaction for both class I and II integron genes contains initial denaturation at 94°C for 5 minutes, followed by 35 main cycles according to [Table/Fig-1] and at the end of the elongation at 72°C for 8 minutes [9]. Finally, PCR products were analysed by electrophoresis and ethidium bromide staining.
The primers and temperature cycles used in the PCR reaction.
| 35 Cycles |
|---|
| Primer | Sequence (5'-3') | Denaturation 94°C | Annealing54°C | Extension 72°C | Product size (bp) |
|---|
| intI1 | F:CAGTGGACATAA GCCTGTTCR:CCCGACGCATAGA CTGTA | 30s | 60s | 2 min | 160 |
| intI2 | F:TTGCGAGTATCCAT AACCTGR:TTACCTGCACTGGA TTAAGC | 30s | 60s | 2 min | 288 |
Ethics
The ethics committee of Kermanshah University of Medical Sciences approved the study protocol (IR.KUMS.REC.1395.250).
Statistical Analysis
Chi-square and Fisher’s-tests were conducted. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 20 and p-values <0.05 were considered statistically significant.
Results
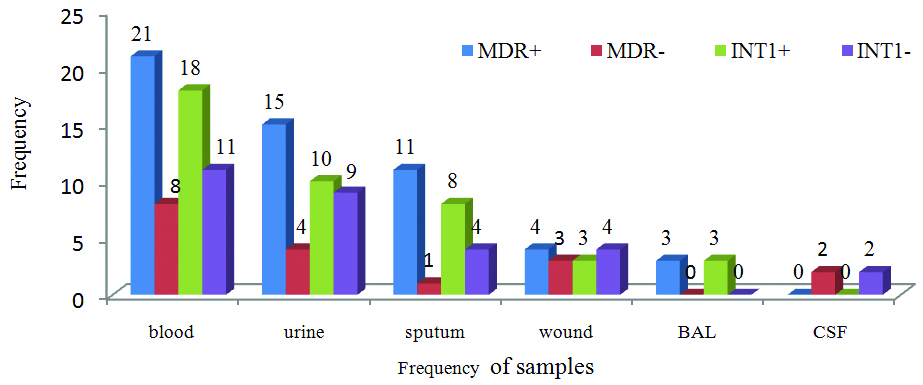
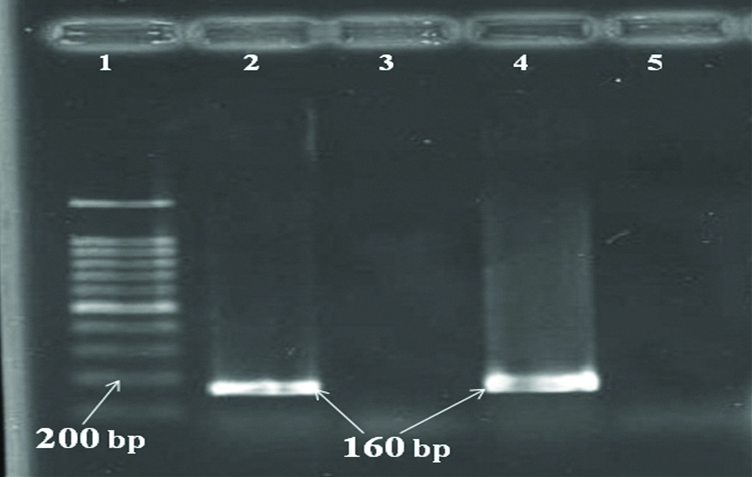
The frequency of the 72 isolates of E.cloacae in females and males was 37 (51.4%) and 35 (48.6%) respectively. The most frequent isolates were in blood sample 29 (40.3%) and urine 19 (26.4%) and the least frequency was in CSF 2 (2.8%). According to the disk diffusion results, the highest antimicrobial resistant was to Ampicillin-clavulanic acid (94.4%) and Cefalexin (69.4%) and the lowest resistance was to Imipenem (9.7%) and Colistin (6.9%) [Table/Fig-2]. Of the 72 isolates of E.cloacae, 54 (75%) were MDR isolates which has highest frequency and was identified in urine and sputum [Table/Fig-3]. The frequency of isolates with INt1 and MDR positive with regard to the age of children is presented in [Table/Fig-4]. Genotypically, the prevalence of class I integron was 58.3% (42 isolates) [Table/Fig-4]. None of the isolates had class II integron. The results of the PCR reaction for identifying the class I integron gene are shown in [Table/Fig-5]. There was a significant relationship between Integron positive isolates and the resistance to Ampicillin-clavulanic acid, Gentamycin and Cotrimoxazole antibiotics [Table/Fig-2].
Association between resistance to antibiotics and the presence of INt1 in isolates of the E.cloacae.
| Antibiotic | Total Resistance No (%) | Positive Integrons 42 isolates | Negative Integrons 30 isolates | p-value |
|---|
| R | I | S | R | I | S | R | I | S |
|---|
| AUG | 68 (94.4) | 0 | 4 (5.6) | 40 | 1 | 1 | 28 | 1 | 1 | <0.001 |
| CFX | 50 (69.4) | 3 (4.2) | 19 (26.4) | 32 | 2 | 8 | 18 | 1 | 11 | 0.118 |
| AK | 35 (48.6) | 3 (4.2) | 34 (47.2) | 30 | 2 | 10 | 17 | 1 | 12 | 0.165 |
| GM | 36 (50) | 6 (8.3) | 30 (41.7) | 24 | 5 | 13 | 14 | 1 | 15 | <0.048 |
| CO | 5 (6.9) | 1 (1.4) | 66 (91.7) | 37 | 0 | 2 | 3 | 3 | 24 | 0.89 |
| NA | 31 (43.1) | 5 (6.9) | 36 (50) | 19 | 3 | 20 | 19 | 2 | 9 | 0.193 |
| CAZ | 38 (52.8) | 2 (2.8) | 32 (44.4) | 24 | 2 | 16 | 19 | 0 | 11 | 0.294 |
| CFM | 40 (55.6) | 9 (12.5) | 23 (31.9) | 25 | 6 | 11 | 20 | 3 | 7 | 0.153 |
| CTX | 37 (51.4) | 6 (8.3) | 29 (40.3) | 22 | 4 | 16 | 15 | 2 | 13 | 0.87 |
| CIP | 35 (48.6) | 3 (4.2) | 34 (47.2) | 19 | 2 | 21 | 16 | 1 | 13 | 0.104 |
| NOR | 30 (41.7) | 1 (1.4) | 41 (56.9) | 18 | 0 | 24 | 12 | 1 | 17 | 0.763 |
| SMX | 49 (68.1) | 8 (11.1) | 15 (20.8) | 26 | 8 | 8 | 23 | 0 | 7 | <0.041 |
| IMI | 7 (9.7) | 9 (12.5) | 56 (77.8) | 6 | 6 | 30 | 1 | 3 | 26 | 0.158 |
| ATM | 29 (40.3) | 3 (4.2) | 40 (55.6) | 16 | 2 | 24 | 13 | 1 | 16 | 0.80 |
| NI | 16 (22.2) | 9 (12.5) | 47 (65.3) | 9 | 6 | 27 | 7 | 3 | 20 | 0.195 |
AUG: Amoxicillin-Clavulanate, CFX: Cefalexin, AK: Amikacin, GM: Gentamycin, CO: Colistin, NA: Nalidixic acid, CAZ: Ceftazidime, CFM: Cefixime, CTX: Cefotaxime, CIP: Ciprofloxacin, NOR: Norfloxacin, SMX: Co-trimoxazole, IMI: Imipenem, ATM: Aztreonam, NI: Nitrofurantoin
The frequency of MDR and INt1 positive E.cloacae isolates in clinical samples.
Frequency of isolates with INt1 and MDR positive according to the age of children.
| Age group (years) |
|---|
| >2 | 2-6 | 7-11 | 12-15 | Total (%) |
|---|
| MDR + | 6 | 21 | 18 | 9 | 54 (75) |
| MDR - | 10 | 5 | 2 | 1 | 18 (25) |
| INt1 + | 7 | 17 | 13 | 5 | 42 (58.3) |
| INt1 - | 9 | 9 | 7 | 5 | 30 (41.7) |
Gel electrophoresis of PCR products of intI, 1- Lader (100 bp), 2- Positive sample (160 bp), 3,5- Negative control, 4- Positive control (160 bp).
Discussion
MDR is a common phenomenon in Enterobacteriaceae. Recently, the prevalence of MDR Enterobacter infection in neonatal intensive care units has been reported [15]. The results of this study demonstrate that E.cloacae is the most frequently isolated organism in urine and blood samples. Various studies have shown that Enterobacter is the most commonly isolated organism from a patient’s blood sample and possibly blood is one of the most suitable habitats for Enterobacter infection [16]. Malekzadegan Y et al., reported that the highest frequency of Enterobacter was detected in blood and urine samples which is in agreement with the current results [2]. In this study, more than 50% of isolates were resistant to Ampicillin clavulanic acid, Cotrimoxazole, Cefalexin, Cefixime, Gentamycin, Ceftazidime and Cefotaxime. The highest antibiotic resistance was seen against Ampicillin- clavulanic acid (94.4%) and Cefalexin (69.4%). Other studies have also reported high levels of resistance to these antibiotics [17,18]. More than 53% of the isolates of Enterobacter were resistant to third-generation Cephalosporins. In similar studies antibiotic resistance spectrum to this pharmaceutical category were reported from 55.56% to 90.1% [2,19,20]. In this regard, present results are consistent with them. In E.cloacae isolates, the lowest resistance was found 6.9% to Colistin and 9.7% to Imipenem like other studies [17,21]. In the treatment of MDR isolates, Carbapenems are often used as the first choice, although in our study, about 9% of isolates were resistant to Imipenem, but the relatively high levels of isolates with intermediate sensitivity can be a sign of reducing the effectiveness of this antibiotic used in the treatment of Enterobacter infection. Present findings suggest that Colistin is an effective agent for MDR Enterobacter isolates along with Colistin, the lowest antibiotic resistance is to Imipenem [22]. Differences in the results of various studies may be due to variations in the spread of antibiotic resistance in different geographical areas as well as the differences in the pattern of antibiotic use. In this study from 72 E.cloacae isolates 54 (75%) was MDR. In Iran, several studies on Enterobacter MDR isolates have been reported from 47.5% to 91.8% that confirmed our results [2,23]. In different studies the high prevalence of class I integron was reported in gram negative bacteria especially MDR isolates [24,25]. In this study, the prevalence of class I integron was 58.3%, with a relatively higher prevalence of class I integron in Enterobacter isolates compared to other studies in the country, including Peymani A et al., 47.5% and Amin M et al., 53% [23,26]. One of the reasons for the higher prevalence of class I integron in our study can be due to the high frequency of isolates of MDR positive. Yu WL et al., and Mokracka J et al., in Poland were two studies in which the prevalence of class I integron in Enterobacter isolates were 65% and 55.1%, respectively [15,27]. In other studies like Ibrahim N et al., in Malaysia, as well as Memariani M et al., in Iran in 2014 for Enterobacteriaceae family members reported the highest prevalence of class I integron for Escherichia coli isolates [28,29]. For example, in other study in Kermanshah the prevalence of class I integron in E.coli isolates, isolated from children was 71.9% [9], this is indicative of high prevalence of class I integron in bacterial isolates in this region, and their distribution among them. In none of the isolates of E.cloacae, Class II integron was observed. In the study of Mokracka J et al., in Poland, the frequency of this class of integron was zero [27]. From the 54 MDR isolates, more than 74% (40 isolate) have class I integron. In a study by Peymani A et al., in Iran and Mokracka J et al., in Poland, a high percentage of MDR isolates had been reported with class I integron, which confirmed the present results. In various studies such as the present study, there is a significant relationship between the prevalence of class I integron and resistance to antibiotics [23,27]. According to various studies some of the antibiotic resistance genes, for example aminoglycosides, cephalosporins, beta-lactamase inhibitors, and others, are transmitted through class I integron [30]. This indicates the effective role of class I integron in generating drug resistance in bacterial isolates.
Limitation
One of the limitations of this study is the sample small size examined. The lack of examination of other classes of integrons for the isolates of E.cloacae is another limitation.
Conclusion
The results of this study demonstrate that there is a high drug resistance in E.cloacae, isolated from children. As a result, due to the role of integrons in resistance to different antibiotics, it is necessary to pay greater attention to their identification and also using appropriate strategies to prevent further development. Due to the emergence of MDR isolates and their serious impact on treatment, antibiotic combinations and prevention of inappropriate use of the antibiotics is recommended.
AUG: Amoxicillin-Clavulanate, CFX: Cefalexin, AK: Amikacin, GM: Gentamycin, CO: Colistin, NA: Nalidixic acid, CAZ: Ceftazidime, CFM: Cefixime, CTX: Cefotaxime, CIP: Ciprofloxacin, NOR: Norfloxacin, SMX: Co-trimoxazole, IMI: Imipenem, ATM: Aztreonam, NI: Nitrofurantoin
[1]. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcomeClin Infect Dis 2004 39(6):812-18.10.1086/42338215472813 [Google Scholar] [CrossRef] [PubMed]
[2]. Malekzadegan Y, Hadadi M, Sedigh Ebrahim-Saraie H, Heidari H, Motamedifar M, Antimicrobial resistance pattern and frequency of multiple-drug resistant enterobacter Spp. at atertiary care hospital in southwest of IranJournal of Krishna Institute of Medical Sciences University (JKIMSU) 2017 6(2):33-39. [Google Scholar]
[3]. Mezzatesta ML, Gona F, Stefani S, Enterobacter cloacae complex: clinical impact and emerging antibiotic resistanceFuture Microbiol 2012 7(7):887-902.10.2217/fmb.12.6122827309 [Google Scholar] [CrossRef] [PubMed]
[4]. Kang CI, Chung DR, Ko KS, Peck KR, Song JH, Clinical predictors of Enterobacter bacteremia among patients admitted to the EDAm J Emerg Med 2012 30(1):165-69.10.1016/j.ajem.2010.09.00320951533 [Google Scholar] [CrossRef] [PubMed]
[5]. de Man P, van Der Veeke E, Leemreijze M, van Leeuwen W, Vos G, van Den Anker J, Enterobacter species in a pediatric hospital: horizontal transfer or selection in individual patients?J Infect Dis 2001 184(2):211-14.10.1086/32201411424021 [Google Scholar] [CrossRef] [PubMed]
[6]. Narayan SA, Kool JL, Vakololoma M, Steer AC, Mejia A, Drake A, Investigation and control of an outbreak of Enterobacter aerogenes bloodstream infection in a neonatal intensive care unit in FijiInfect Control Hosp Epidemiol 2009 30(8):797-800.10.1086/59824019552517 [Google Scholar] [CrossRef] [PubMed]
[7]. Peymani A, Naserpour Farivar T, Reza Najafipour R, Mansouri S, High prevalence of plasmid-mediated quinolone resistance determinants in Enterobacter cloacae isolated from hospitals of the Qazvin, Alborz, and Tehran provinces, IranRev Soc Bras Med Trop 2016 49(3):286-91.10.1590/0037-8682-0454-201527384824 [Google Scholar] [CrossRef] [PubMed]
[8]. Liu J, Zeng T, Su G, Lin LY, Zhao Y, Yang WQ, The dissemination mode of drug resistant genes in Enterobacter cloacaeIndian J Med Microbiol 2015 33:S87-92.10.4103/0255-0857.15089925657163 [Google Scholar] [CrossRef] [PubMed]
[9]. Vaziri S, Abiri R, Mansouri F, Alvandi A, Azizi M, Hasanvand B, The molecular investigation of Class 1 and 2 Integrons among the Escherichia Coli Isolated from urine samples of children in Imam Hospital, Kermanshah City, Iran, in 2016J Isfahan Med Sch 2017 35(446):1171-77.[In Persian] [Google Scholar]
[10]. Gillings MR, Integrons: past, present and futureMicrobio Mol Biol Rev 2014 78:257-77.10.1128/MMBR.00056-1324847022 [Google Scholar] [CrossRef] [PubMed]
[11]. Japoni S, Japoni A, Farshad S, Ali AA, Jamalidoust M, Association between existence of integrons and multi-drug resistance in Acinetobacter isolated from patients in southern IranPol J Microbiol 2011 60(2):163-68. [Google Scholar]
[12]. Collis CM, Kim MJ, Partridge SR, Characterization of the class 3 integron and the site-specific recombination system it determinesJ Bacteriol 2002 184:3017-26.10.1128/JB.184.11.3017-3026.200212003943 [Google Scholar] [CrossRef] [PubMed]
[13]. Hall RM, Collis CM, Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombinationMol Microbial 1995 15(4):593-600.10.1111/j.1365-2958.1995.tb02368.x7783631 [Google Scholar] [CrossRef] [PubMed]
[14]. Clinical and Laboratory Standards Institute, Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement, CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA, 2014 [Google Scholar]
[15]. Yu WL, Cheng HS, Lin HC, Peng CT, Tsai CH, Outbreak investigation of nosocomial Enterobacter cloacae bacteraemia in a neonatal intensive care unitScand J Infect Dis 2000 32(3):293-98.10.1080/0036554005016594710879601 [Google Scholar] [CrossRef] [PubMed]
[16]. Souza Lopes AC, Rodrigues JF, Cabral AB, da Silva ME, Leal NC, da Silveira VM, Occurrence and analysis of irp2 virulence gene in isolates of Klebsiella pneumoniae and Enterobacter spp. from microbiota and hospital and community-acquired infectionsMicrob Pathog 2016 96:15-19.10.1016/j.micpath.2016.04.01827133266 [Google Scholar] [CrossRef] [PubMed]
[17]. Ghorashi Z, Ghorashi S, Soltani-Ahari H, Nezami N, Demographic features and antibiotic resistance among children hospitalized for urinary tract infection in northwest IranInfection and Drug Resistance 2011 4:171-76.10.2147/IDR.S2417122114509 [Google Scholar] [CrossRef] [PubMed]
[18]. Mahmoudi S, Mahzari M, Banar M, Pourakbari B, Haghi Ashtiani MT, Mohammadi M, Antimicrobial resistance patterns of Gram-negative bacteria isolated from bloodstream infections in an Iranian referral paediatric hospital: A 5.5-year studyJ Glob Antimicrob Resist 2017 11:17-22.10.1016/j.jgar.2017.04.01328729206 [Google Scholar] [CrossRef] [PubMed]
[19]. Nematolahi S, Mosadegh A, Mardaneh J, Poorabbas B, Identification of ESBL-producing and blaSHV gene harboring Enterobacter spp. isolated from bloodstream infections of hospitalized patients during 10 years in south of Iran (Shiraz)Iran South Med J 2016 19(4):536-48.[In Persian]10.18869/acadpub.ismj.19.4.536 [Google Scholar] [CrossRef]
[20]. Behzadnia S, Davoudi A, Rezai MS, Ahangarkani F, Nosocomial infections in pediatric population and antibiotic resistance of the causative organisms in north of IranIran Red Crescent Med J 2014 16(2):e1456210.5812/ircmj.1456224719744 [Google Scholar] [CrossRef] [PubMed]
[21]. Haghi-Ashteiani M, Sadeghifard N, Abedini M, Soroush S, Taheri-Kalani M, Etiology and antibacterial resistance of bacterial urinary tract infections in children’s medical center, Tehran, IranActa Medica Iranica 2007 45(2):153-57. [Google Scholar]
[22]. Dalhoff A, Global fluoroquinolone resistance epidemiology and implictions for clinical useInterdiscip Perspect Infect Dis 2012 976273:1-37.10.1155/2012/97627323097666 [Google Scholar] [CrossRef] [PubMed]
[23]. Peymani A, Farivar TN, Sanikhani R, Javadi A, Najafipour R, Emergence of TEM, SHV and CTX-M-extended spectrum beta-lactamases and class 1 integron among Enterobacter cloacae isolates collected from hospitals of Tehran and Qazvin, IranMicrob Drug Resist 2014 20:424-30.10.1089/mdr.2013.019124684320 [Google Scholar] [CrossRef] [PubMed]
[24]. Mirnejad R, Mostofi S, Masjedian F, Antibiotic resistance and carriage class 1 and 2 integrons in clinical isolates of Acinetobacter baumannii from Tehran, IranAsian Pac J Trop Biomed 2013 3:140-45.10.1016/S2221-1691(13)60038-6 [Google Scholar] [CrossRef]
[25]. Mehdipour Moghaddam MJ, Mirbagheri AA, Salehi Z, Habibzade SM, Prevalence of class 1 integrons and extended spectrum beta lactamases among multi-drug resistant escherichia coli isolates from north of IranIran Biomed J 2015 19(4):233-39. [Google Scholar]
[26]. Amin M, Dibachi S, Shahin M, Prevalence of class 1 integrons and plasmid-mediated qnr-genes among Enterobacter isolates obtained from hospitalized patients in Ahvaz, IranInfez Med 2017 25(4):351-57. [Google Scholar]
[27]. Mokracka J, Koczura R, Pawłowski K, Kaznowski A, Resistance patterns and integron cassette arrays of Enterobacter cloacae complex strains of human originJournal of Medical Microbiology 2011 60:737-43.10.1099/jmm.0.027979-021330416 [Google Scholar] [CrossRef] [PubMed]
[28]. Ibrahim N, Wajidi MF, Yusof MY, Tay ST, The integron prevalence of extended-spectrum betalactamase producing enterobacterial isolates in a Malaysian teaching hospitalTrop Biomed 2011 28(3):668-71. [Google Scholar]
[29]. Memariani M, Najar Peerayeh S, Shokouhi Mostafavi SK, Zahraei Salehi T, Detection of class 1 and 2 integrons among enteropathogenic Escherichia coli isolatesArch Pediatr Infect Dis 2014 2(4):e1637210.5812/pedinfect.16372 [Google Scholar] [CrossRef]
[30]. Peymani A, Naserpour Farivar T, Ghoraiian P, Najafipour R, Association between class 1 integrons and multidrug resistance pattern among Enterobacter spp. isolated from Qazvin and Tehran teaching hospitalsJ Qazvin Univ Med Sci 2014 18(2):30-38.[In Persian]10.17795/bhs-22085 [Google Scholar] [CrossRef]