Obstructive jaundice is a common presentation seen in biliary lesions encountered in clinical practice in India. It may be due to intrahepatic and extrahepatic causes. Extrahepatic cholestasis, characterized by dilated bile ducts, is often caused by a bile duct stricture or stone or malignancy [1]. Most of the literature published from Northern India clearly shows that majority of patients presenting in large set-ups have malignant lesions as cause of obstructive jaundice [2]. Reported incidence of malignant lesions ranges from 63.3% to 75% in comparison to benign cases [2-4]. Most of these cases undergo either diagnostic or therapeutic ERCP. Direct visualisation by ERCP still remains the gold standard in evaluation of the extrahepatic bile duct.
Biopsy is usually difficult and a very small tissue piece is submitted for histological evaluation which might not be representative of the diseased area in case of malignant strictures. However, properly performed biliary brush cytology can give better output in these cases. The advantages offered by biliary brush cytology over biopsy are that more area is sampled and a flexible brush can be negotiated in stricture area beyond the scope. However, skill of the endoscopist and the time taken to make smears plays a major role in the application of biliary brushing in interpretation of biliary strictures.
Present study emphasises the role of doing brush cytology at the time of ERCP procedure which is inadvertently performed in most of the cases of malignant biliary stricture, so that patient is not subjected to any subsequent procedure for diagnosis and subtyping of tumour. In addition, the sensitivity of the procedure can be increased, if strict cytological criteria are followed and also correct procedure of smearing is followed.
Materials and Methods
Study sample: This was a prospective study done over a period of one year, we analysed 40 patients reporting to Department of Surgical Gastroenterology of King George Medical University, Lucknow, Uttar Pradesh, India. Patients who presented with signs and symptoms of obstructive jaundice (due to biliary strictures) with clinical suspicion of malignancy were included in the study. Diagnosis of malignancy was confirmed clinically and radiologically or by clinical course of the disease or histopathology or fine needle aspiration cytology. In this study, some of the patients showed rapid increase in size of mass on radiology and showed clinical signs of malignancy. All patients with benign causes of obstructive jaundice or any other associated malignancy were excluded. Five cases of benign causes of biliary stricture were taken as controls to define baseline benign cytomorphology in the smears and for standardization of the technique. Smear staining and analysis of data was done in Department of Pathology. Ethical committee clearance was taken.
Methods: Patients were assessed clinically and demographic data of the patients were recorded. Clinical and biochemical parameters were properly recorded for each case (including detailed clinical history and Liver function test). Radiological investigations including ultrasound, computed tomography and cholangiogram findings were also noted. ERCP procedure was performed using a standard video duodenoscope. After visualisation of ampulla, endoscopic sphincterectomy was performed using a papillotome introduced over a guide wire. Under radiographic guidance using a contrast fluid, bile duct strictures were localised. Strictures were brushed with rapid to and fro movements using a double lumen brush. The procedure was performed by experienced gastro-surgeon under strict aseptic protocol and after well informed written consent from the patient. Adequate brush cytology sample could be obtained in all the study subjects. Cytosmears were prepared by experienced pathologist in the endoscopy procedure room.
Cellular material adherent to the brush was directly transferred to the glass slide and five smears were prepared and stained by rapid Papanicolau (2 smears), Haematoxylin and eosin (2 smears) and Giemsa stain (1 smear). Detailed cytological evaluation was done and recorded. Statistical analysis was done by SPSS 23 version software.
Cytomorphological evaluation: Cytological smears were assessed for described cytological characteristics in detail. Morphological features assessed included: 1) cellularity; 2) background; 3) pattern of cell distribution; 4) polarity; 5) nuclear size; 6) nuclear to cytoplasmic ratio; 7) nuclear variation >(x2 or x4); 8) nuclear shape; 9) nuclear outline; 10) chromatin; 11) mitosis; 12) amount of cytoplasm; 13) background atypical cells; and 14) multinucleate cell. Other cytological features like nuclear grooving, convolutions, notches and moulding and necrosis were also studied. The nuclear size was comparable to size of RBC and nuclear variation was compared to a benign glandular cell from benign smears. Nuclear cytoplasmic ratio was reported as increased when it was greater than 2:1 or when the nucleus constituted more than 50% of entire cell volume. The smears were seen by two independent pathologist and final consensus was taken by a third pathologist in case of discrepancy. The smears were classified as benign, reactive, malignant and Not Otherwise Specified (NOS)-suspicious. For purpose of analysis NOS-suspicious smears were considered to be positive and reactive as negative. The patients were followed up clinically and radiologically. Diagnostic sensitivity of the brush cytology procedure was assessed along with correlation with the demographic and cytological characteristic. Sensitivity of the procedure was calculated by the formula; sensitivity=true positive cases/(true positive+false negative) x100.
Results
Total 40 malignant cases underwent transpapillary brush cytology during the study period. The mean age of the patients was 54 years. Males were 13 and females were 27(M:F ratio=1: 2.1). Rest were confirmed by clinical follow-up and radiology. Carcinoma gall bladder was the most common malignancy seen in our cases (62.5%; 25/40) followed by cholangiocarcinoma (20%; 8/40), periampullary carcinoma (12.5%; 5/40) and pancreatic carcinoma (5%; 2/40) respectively. In 22 out of 40 cases histological confirmation for malignancy was available. In 18/22 cases of carcinoma gall bladder USG guided aspiration cytology was also available and was positive for malignant cells.
Amongst the biochemical investigations, the mean serum bilirubin level was 11.32 mg/dL and mean alkaline phosphatise level was 1595 IU/L. These levels were consistent with laboratory parameters of obstructive jaundice. Patents did not have any procedure related complications while undergoing the procedure.
Biliary brush cytology: Ninety five percent of the cases sampled by biliary brush (38/40) had adequate cellular material for interpretation in the first brushings, only 5% (2/40) were inadequate. The procedure was repeated as there was strong radiological and clinical suspicion of malignancy and adequate samples were obtained in them. The cytological smears were classified as benign, reactive, NOS-suspicious and malignant. One case was sampled thrice during course of the disease in which first two smears revealed reactive cytology and last smear was malignant. The final diagnosis was considered. The procedure was repeated as the patient was clinically deteriorating and the tumour was increasing in size radiologically. Out of 40 malignant cases, the cytological smears of eight were classified as benign, five as reactive, five as NOS-suspicious, and 22 as malignant. For purpose of analysis NOS-suspicious smears were considered to be positive and reactive as negative. Diagnostic sensitivity of brush cytology for detection of all malignancies was 67.5% (27/40); 56% cases (14/25) of carcinoma gall bladder, 87.5% cases of cholangiocarcinoma (7/8), 80% cases of periampullary carcinoma (4/5) and 100% of pancreatic ductal adenocarcinoma (2/2) were diagnosed by brush cytology [Table/Fig-1].
Sensitivity of biliary brush cytology.
| Clinical diagnosis | Number of cases | Detected | Not detected | Sensitivity (%) |
|---|
| Carcinoma gall bladder | 25 | 14 | 11 | 56 |
| Cholangiocarcinoma | 8 | 7 | 1 | 87.5 |
| Periampullary carcinoma | 5 | 4 | 1 | 80 |
| Pancreatic carcinoma | 2 | 2 | 0 | 100 |
| Total | 40 | 27 | 13 | 67.5 |
Cellular details of all the smears are summarized in [Table/Fig-2]. The benign smears were low in cellularity with cells disposed in monolayer sheets of regular round epithelial cells, nuclear to cytoplasmic ratio was normal and cytoplasm was adequate. No atypical cells, giant cells, mitosis or necrosis was seen. Reactive smears had moderate to high cellularity with haemorrhage and acute inflammation in the background. Cells were disposed in sheets and clusters with mild pleomorphism, polarity was maintained and nuclear crowding was absent [Table/Fig-3]. The malignant smears had moderate to high cellularity with haemorrhage and acute inflammation in background. Cells were disposed in sheets and clusters with pseudopapillae and acini formation. Polarity was lost with increased nuclear cytoplasmic ratio. The tumour cells were round to oval, some were spindled. Nuclear size was >1.5 RBC. Reactive cell clusters were seen in five of the malignant smears. Necrosis was present in 18/22 malignant smears. Smears from five cases displayed cytomorphology intermediate between reactive and malignant smears. They were classified as NOS -suspicious. The smears were cellular with cell disposed in sheets and clusters. Nuclear clumping was present but the nuclear membrane was regular, nuclear variability intermediate (between x2-3) with nucleolar prominence in one case. No mitosis, multinucleate cells were seen in the background. 4/22 malignant smears had significant inflammation in the background [Table/Fig-4].
Detailed cytological feature evaluation in each cytological category.
| Benign smear (n=8) | n=8 | Reactive smear (n=5) | n=5 | Malignant (n=22) | n=22 | NOS-suspicious (n=5) | n=5 |
|---|
| Cytologic features | Category |
| Cellularity | Low | 8 | ModerateHigh | 32 | ModerateHigh | 1012 | ModerateHigh | 32 |
| Background | Clear with slight hemorrhage | 8 | HemorrhageAcute inflammation | 53 | HemorrhageAcute inflammation | 188 | HemorrhageAcute inflammation | 41 |
| Cell disposed in | Monolayer sheets(1-3)Benign columnar cells<2Cluster of <5 cells | 888 | Sheets(>5)clusters | 55 | Sheets(>5)ClustersPseudopapillae Acini formation | 151787 | Sheets(>5)ClustersPseudopapillae Acini formation | 2311 |
| Polarity | Maintained | 8 | Maintained | 4 | Lost | 19 | Maintained | 3 |
| Nuclear size | >1.5 RBC size | 0 | >1.5 RBC size | 0 | >1.5 size of RBC | 17 | >1.5 size of RBC | 4 |
| Nuclear to cytoplasmic ratio | Normal | 8 | Normal | 4 | Increased | 20 | Increased | 3 |
| Shape | Oval to round | 8 | Oval to round | 5 | Oval to roundAngulatedGroovingMoulding | 41054 | Oval to round to elongated | 4 |
| Margins | Regular | 8 | Regular | 5 | Irregular | 21 | Irregular | 3 |
| Variation | Absent | 8 | <(x2) | 5 | >(x2)>(x4) | 1012 | Between (x2-3) | 5 |
| Chromatin | Fine | 8 | Fine | 5 | Hyperchromatic | 12 | Clumped | 4 |
| Nucleoli | Absent | 8 | Absent | 5 | Present | 21 | Present occasionally | 4 |
| Cytoplasm | Pale eosinophilic | 8 | Pale eosinophilic | 5 | ModerateScant | 913 | Moderate | 5 |
| Background atypical cells | Absent | 8 | Absent | 5 | Present | 8 | Present | 2 |
| Giant cells | Absent | 8 | Absent | 5 | Present | 7 | Absent | 5 |
| Mitosis | Absent | 8 | Absent | 5 | Present | 4 | Present | 5 |
| Necrosis | Absent | 8 | Absent | 5 | Present | 18 | Absent | 5 |
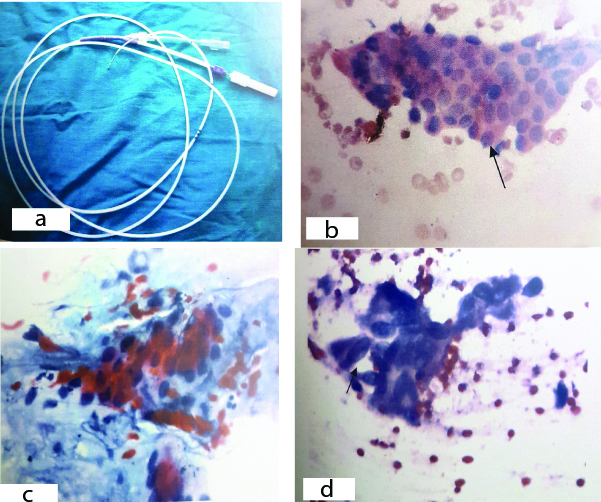
a) Double lumen 8 freanch brush which increased yield in study. b) Benign smear showing a benign sheet (arrow), (H&E X 400). c) Smear showing nuclear variability (<X2), nuclear membrane is regular (Pap X 100). d) Smear showing nuclear grooving arrow, (Pap X 400).
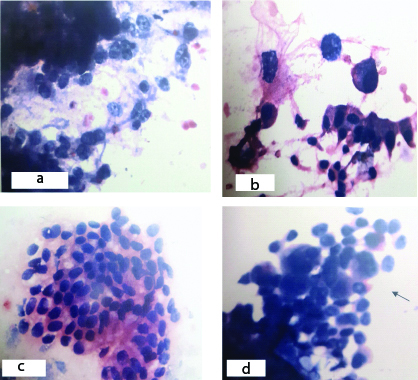
a) Mailgnant smears showing nuclear varibility (Pap X400). b) Cells showing increased nucleo cytoplasmic ratio (Pap X400). c) Atypical cells forming sheets (HE X400). d) Smear showing multinucleate cells (Pap X400).
Discussion
Early diagnosis of malignant biliary strictures still remains a challenge. Most of the patients present late when palliative treatment like stenting is the mainstay of treatment. Usually the malignancy is locally advanced with or without distant metastases and curative resection cannot be done in such patients. Even then an accurate tissue diagnosis is needed in both resectable and non-resectable tumours to plan chemotherapy [5].
Biliary and pancreatic duct lesions are not always readily accessible to biopsy, and cytological techniques have become the initial diagnostic modality in many cases [6,7].
Amongst the diagnostic procedures, percutaneous radiologically guided fine needle aspiration is a very accurate technique in pancreatobiliary malignancies but for this there must be a distinct mass lesion for adequate sampling. Other methods like bile, pancreatic and duodenal fluid aspirates have low sensitivity and specificity because of poor cellular preservation [8]. Brush cytology performed during ERCP has now become the preferred initial method of diagnosis in many patients with pancreatico–biliary strictures. The technique allows sampling from most sites within the pancreatic and biliary duct systems. Well prepared and sampled specimens usually have well preserved cellular samples suitable for cytological analysis [9,10].
In the present study, malignancy was common in older age group. Furthermore, women were affected more frequently than men. Similar observations were seen by other authors [11,12].
Modest diagnostic sensitivity is recorded in most studies to date (33-78%; mean 42%) and negative predictive value, despite high test specificity (90-100%). A comparison between various studies with respect to sensitivity, specificity, negative predictive value and positive predictive value is summarised in [Table/Fig-5] [13-22].
Summary of studies comparing diagnostic efficacy of Endscopic retrograde Cholangiopancreatography guided brush cytology.
| Author | Year | Total No. of patients | No. of patients with cancer | No. of cases detected by Brush cytology | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|
| Lee G J et al., [13] | 1995 | 149 | 106 | 40 | 37 | 100 | 100 | 39 |
| Ponchon T et al., [14] | 1995 | 204 | 127 | 45 | 35 | 97 | 96 | 44 |
| Pugliese V et al., [15] | 1995 | 94 | 64 | 35 | 54 | 100 | 100 | 50 |
| Glasbrenner B et al., [16] | 1999 | 78 | 57 | 32 | 56 | 90 | 94 | 43 |
| Mansfield JC et al., [17] | 1997 | 43 | 41 | 17 | 42 | 100 | 100 | 8 |
| Jailwala J et al., [18] | 2000 | 133 | 104 | 31 | 30 | 100 | 100 | 28 |
| Macken E et al., [19] | 2000 | 106 | 62 | 35 | 57 | 100 | 100 | 62 |
| Stewart CJR et al., [20] | 2001 | 406 | 246 | 147 | 60 | 98 | 98 | 61 |
| Fogel EL et al., [21] | 2006 | 102 | 94 | 28 | 30 | - | - | - |
| Shieh FK et al., [22] | 2014 | 32 | 32 | 25 | 78 | - | - | - |
| Present study | 2018 | 40 | 40 | 27 | 67.5 | - | - | - |
In present study the procedure had a moderate diagnostic sensitivity of 67.5%. The procedure was performed by a well-trained gastro-surgeon and protocol based evaluation of smears was done. The reasons for low sensitivity in most of the studies may be due to sampling and interpretative differences. Sampling errors might occur when tumours at these sites show a predominantly submucosal spread, with limited or absent surface epithelial abnormality [23,24]. Strictures might also be caused by external compression like by lymph node metastasis. The site of the tumour might also affect the sensitivity. Interpretative errors are more likely to occur in well differentiated carcinomas, in which the cytological abnormality may be minimal [25].
Several studies have shown that diagnostic accuracy is greatest for ampullary neoplasms, intermediate for cholangiocarcinoma, and lowest for pancreatic carcinoma, particularly for tumours in the pancreatic tail [26]. Although in this study, detection rate was 80% in ampullary carcinoma cases, 87.5% in cholangiocarcinoma, 100% in pancreatic ductal adenocarcinoma and 56% in carcinoma gall bladder causing obstruction.
An interesting finding found in present study was that an increase in detection rate was found during later half of study. Possible explanations are the use of double lumen brush instead of single lumen later in the study and increase in surgeon’s expertise in taking the sample. In addition, more of forward movement of the brush was done to increase the cellular yield. Also, removing the brush and catheter together improved cancer detection compared with pulling the brush through the catheter sheath.
As per present study cytological features which were found more consistent with malignancy were high cellularity, loss of polarity, hyperchromatic nucleus with clumped chromatin, nuclear crowding, irregular nuclear membrane and nucleolar prominence. Nuclear variability, background atypical cells and multinucleate cells were also seen in smears which were reported as positive.
Cytological features like inflammation had a similar frequency in benign and malignant bile duct brushings, making it less useful in the accurate identification of malignant brushings. However, it should be noted that acute inflammation should not be ignored in all cases, particularly when associated with other concerning malignant characteristics. Five of our malignant smears had inflammation in the background and few clusters were atypical.
In an effort to improve the diagnostic accuracy of cytologic examination, several investigators have proposed specific diagnostic criteria for malignancy.
Avadhani V et al., had seven reviewers for bile duct brushing specimens who examined strength of several well established cytological characteristic in predicting malignancy [27]. They studied change in chromatin pattern, nuclear irregularity, pleomorphism, 2- cell population and three dimensional clusters were helpful in accurately identifying malignancy in these specimens and were present in more than 50% cases. Okonkwo AM et al., studied strict morphologic criteria: major (nuclear contour, chromatin pattern) and minor (polarity, cell types, nuclear size, nuclear grooves, nucleoli, mitosis, nuclear/cytoplasmic ratio). Irregularities in nuclear membrane and abnormal chromatin pattern were the most consistently useful features correlating with malignancy, which are similar to the criteria we have studied. Their study was based on conventional smears which is also similar to this study [28].
However, Renshaw AA et al., observed that overall assessment of malignancy based on degree of atypia was more reproducible than any set of criteria and also resulted in higher sensitivity for malignancy with small decrease in specificity [29].
Regarding inflammation in the background of malignant smears, it has been demonstrated that pancreatic ductal adenocarcinomas, particularly its micropapillary and undifferentiated subtypes, may demonstrate marked intra-epithelial infiltration in pancreatic ductal adenocarcinoma which may involve the bile ducts resulting in ‘positive’ bile duct brushings [30].
Lack of obvious features of carcinoma should be prompt the surgeon to do repeat sampling to prompt a conclusive diagnosis of malignancy. Two cases in our study had repeated aspirations as they had strong clinical and radiological suspicion of malignancy.
Present study did not have any procedure related complications. In a series of 223 consecutive biliary strictures sampled by brush cytology, Ponchon T et al., reported one retroperitoneal bile duct perforation related to the brushing. The patient was treated with placement of a biliary stent and remained asymptomatic. These brushings were obtained without the use of a guide wire; when brushing is performed over a guide wire, the likelihood of this adverse event is low [14].
Considering current diagnostic aids to brush cytology, immunohistochemistry is another useful adjuvant study in bile duct brushing assessment which is been recently studied. In a recent study, over 50% of biliary cancers showed a maspin +/IMP3+/S100P+/pVHL − staining profile, and 20% showed a maspin+/IMP3 − /S100P+/pVHL− profile [31]. However immunohistochemistry lacks specificity. A newer pancreato-biliary tract specific FISH probes have been identified that targets 1q21, 7p12, 8q24, and 9p21, with a sensitivity of 65% and specificity of 93% [32]. However, FISH poses technical and financial challenges.
The search for improvements in diagnostic accuracy of bile duct brushings has led some to suggest triple testing (brush cytology, fluorescence in situ hybridization and forceps biopsy) which has 82% sensitivity, 100% specificity, 100% positive predictive value, and 87% negative predictive value as compared to brushing alone [33]. However, these studies had sufficient specimen cellularity for additional testing, which is often not the case in clinical practice and also most centres lack facilities and molecular tests are not cost effective.
Limitation
Furthermore, current study also has few limitations, adequate number of benign cases was not included in the study, therefore, specificity could not be calculated. Biochemical investigations from benign cases could not be compared with the malignant cases and any cut-off value to differentiate between benign and malignant cases could not be assessed.
Conclusion
To conclude, the results of the present study clearly show that biliary brushing is good and cost effective if these guidelines are properly followed: 1) smears are appropriately prepared by a double lumen brush with more of forward movement rather backwards during sample collection; 2) strict cytological criteria for interpretation and reporting is followed. The limitations of the technique must be recognised, brush cytology is useful as the initial investigation of patients with suspected pancreatico–biliary neoplasia and the present study fulfils the need to study individual morphological characteristics and prove their diagnostic utility.
Future recommendations are for development of strategies to improve lesion targeting and better retrieval of cytology specimens with higher quality and also studies with larger number of cases along with multi-reviewer analysis adds to calculation of diagnostic efficacy of the procedure.