Comparision of Efficacy of Low Dose (25 mg) with High Dose Diclofenac (50 mg) in Management of Postoperative Pain after Periodontal Flap Surgery: A Randomised Clinical Trial
Kshitij K Karmkar1, Dilip D Khambete2, Abhijit N Gurav3, Rahul A Patil4, Abhijeet R Shete5, Sumit S Shetgar6, Nibha S Kulkarni7, Swapneel C Bodele8
1 PG Student, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
2 Professor and Head, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
3 Professor, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
4 Reader, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
5 Reader, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
6 Lectrur, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
7 PG Student, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
8 PG Student, Department of Periodontology, Tatyasaheb Kore Dental College and Hospital, Kolhapur, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kshitij K Karmkar, PG Student, Department of Periodontology, Tatyasaheb Kore Dental College and Research Centre, New Pargaon, Taluka, Hatakangaledist-416137, Kolhapur, Maharashtra, India.
E-mail: kkarmkar15@gmail.com
Introduction
The perception of pain is highly subjective and varies substantially among individuals. Diclofenac is powerful NSAID and is associated with adverse effect, like gastric irritability.
Aim
To compare a low dose Diclofenac (25 mg) with Diclofenac (50 mg) in postoperative pain management after periodontal flap surgeries.
Materials and Methods
Study included 20 patients with chronic periodontitis (17-55 years), scheduled for open flap debridement surgery on at least two quadrants >1 week apart. Study group was divided into two groups; one group received low dose Diclofenac (25 mg Diclofenac and 325 mg paracetamol), BID for three days, whereas the other group received Diclofenac (50 mg Diclofenac and 325 mg paracetamol), BID for three days. The pain assessment of the subjects was carried out by VAS and pain assessment questionnaire.
Results
The mean pain scores in both groups were obtained by VAS. After comparing those pain scores of both groups by independent t-test and repeated measures of ANOVA test, statistically significant (p<0.05) results were found between both groups at day 1,2 and 3 but less gastric discomfort was observed with Diclofenac 25 mg group.
Conclusion
Diclofenac 25 mg can be used efficiently in controlling postoperative pain after open flap debridement, especially in patients with gastric discomfort.
Cyclooxygenase, Drug administration, Post operative pain management
Introduction
Pain after periodontal surgical procedures is a common manifestation. The perception of pain is highly subjective and varies substantially among individuals [1]. Many factors affect pain perception, such as the nature, duration and extent of the surgery and psychological aspects (e.g., stress and anxiety) [2,3]. Pain after periodontal surgery is an example of acute dental pain of mild to moderate severity [4]. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) have a significant advantage in the control of pain after periodontal or oral surgical procedures [5-7].
"Diclofenac" is the most commonly prescribed NSAID worldwide and has been available in the United States since 1988 [8]. Diclofenac inhibits the activity of Cyclooxygenase (COX)-1, which has been associated with the gastrointestinal Adverse Effects (AEs) associated with NSAIDs and COX-2, the inhibition of which has been associated with NSAID therapeutic efficacy but is related with an increased risk of cardiovascular adverse effects [9,10]. Diclofenac has been reported to achieve analgesia at concentrations associated with clinically relevant inhibition of the COX-2 enzyme (IC80; the drug concentration that produces 80% inhibition), with a lesser degree of COX-1 inhibition [11]. However, diclofenac products are often dosed supra therapeutically. For example, the commonly used diclofenac dosage of 50 mg Three Times Daily (TID) achieves 90% inhibition of COX- 2 over eight hours after dosing, with only partial inhibition of COX-1 (49.5%). Consequently, lowering the dose would continue to provide analgesia with the potential of a reduced risk for AEs [12]. Large-scale pharmaco epidemiologic studies [13-15] have corroborated findings from clinical studies of toxicity risks with NSAID use. Additional studies have examined the tolerability risks with the use of low versus high doses of NSAIDs and found increased risks in patients receiving high-dose NSAIDs compared with those in patients receiving low doses of these agents [16-21]. Based on the pooled evidence, regulatory agencies around the world, including the US Food and Drug Administration [22], the European Medicines Agency [23], and Health Canada [24], have recommended the use of NSAIDs at the lowest effective dosage and for the shortest duration.
To help physicians and patients to better comply with the recommendation of the use of the lowest effective doses of NSAIDs. Recently a lower-dose submicron version of diclofenac, was created using SoluMatrix Fine Particle Technology (Iroko Pharmaceuticals, Philadelphia, PA). SoluMatrix diclofenac capsules (25 mg) are equivalent to a 50% lower than the normal diclofenac dose. This novel, nonselective NSAID was recently approved by the US Food and Drug Administration for the treatment of acute and chronic pain [22].
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms. Many clinical trials have suggested Diclofenac 50 mg alleviates the postoperative pain, the aim of the present study was to compare a low dose Diclofenac (25 mg) with Diclofenac (50 mg) in postoperative pain management after periodontal flap surgical procedure.
Materials and Methods
The present split-mouth, single-blind randomised clinical trial (NCT03519152) was carried out in the Department of Periodontology and source of the patients was from the outpatient section of Tatyasaheb Kore Dental College and Research Centre, New Pargaon, Maharashtra, India. The ethical clearance was taken from Ethical Committee of the Institute. Patients were enrolled in the study between November 2016 and June 2017. Based on the power of study that was 80% and alpha of 0.05 with SD of ±1.47, authors required 36 quadrants. Considering 10% dropout, sample size was finalised as 20. The study included 20 patients (14 males and 6 females) with generalised chronic periodontitis.
With age ranging from 17-55 years, the selected patient had at least 20 natural teeth, and with no history of previous periodontal therapy preceding six months of study. Medically compromised patients, pregnant and lactating mothers, smokers and alcoholics, those with a history of taking anticoagulant therapy, patient reporting intake of steroidal or non-steroidal anti-inflammatory drugs (previous three months) or antibiotics in previous six month were excluded from the study [Table/Fig-1]. Patient with known hypersensitivity to Diclofenac and gastric diseases were also not considered. The protocol of the study was explained to each patient, and informed consent was obtained after explanation of the study. A split-mouth design was used. A total of 40 quadrants in 20 patients (two quadrants in each patient) were operated. Complete medical evaluations of all the patients were done to rule out any systemic conditions.
Flowchart of study design.
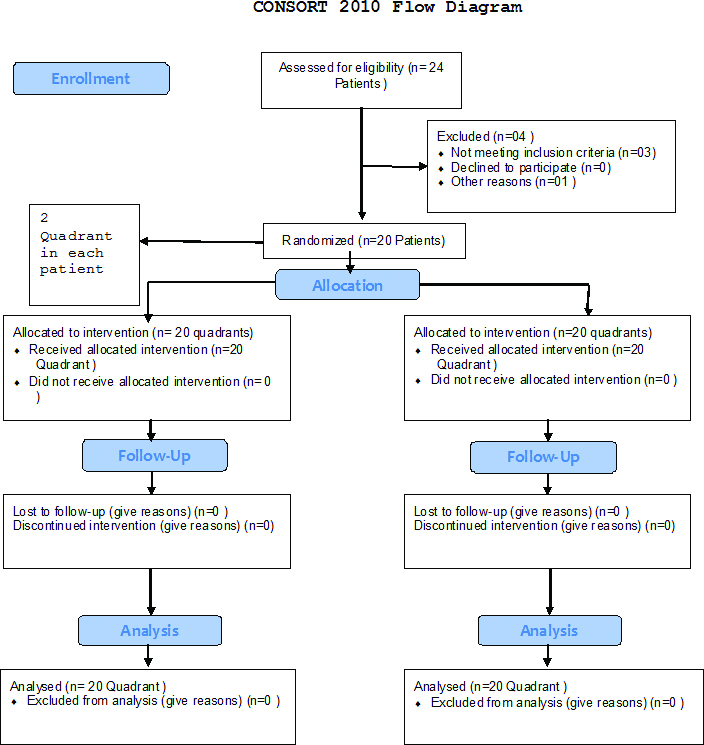
All patients were scheduled for open flap debridement surgery on at least two quadrants >1 week apart. Each quadrant was randomly allocated (coin test) a different medication regimen for postoperative pain control and the patient was kept blinded to which medication they received during the treatment in each quadrant. So in one patient two quadrants were operated, one quadrant received low dose Diclofenac tablets {(DYNAPAR LD, TROIKAA PHARMACEUTICALS LTD.,) 25 mg Diclofenac and 325 mg paracetamol}, BID for three days, whereas the other operated quadrant received Diclofenac {(DICLOMOL, WIN- MEDICARE) 50 mg Diclofenac and 325 mg paracetamol}, BID for three days. A flap was raised under local anaesthesia (2% lignocaine with 1:80,000 epinephrine). In both quadrants, same technique of anaesthesia was employed. The location and the extent of surgery, volume of the local anaesthesia given, and time required to perform the surgical procedure were noted in the patient file [Table/Fig-2]. Patients were instructed to complete VAS chart once in the morning and once in evening for three days with the gap of eight hours in between and were recalled on 4th day and asked about any discomfort noted during following postoperative days in the form of questionnaire. For measuring the clinical pain intensity the patients were provided with the Visual Analog Scale (VAS) [Table/Fig-3]. The VAS consists of a 10 cm line anchored by two extremes: no pain and pain that could not be more severe. Patients were asked to make a mark on the line representing their level of perceived pain.


Statistical Analysis
Data were analysed using statistical software. The p-value was set at 0.05 for all tests. The post-surgical pain parameters were presented as mean±SD and were compared using the independent t-test. For the intra group analysis repeated measures of analysis of variance (ANOVA) test was used.
Results
Twenty acquiescent patients {14 males (70%) and 6 females (30%)} between the ages of 17 and 55 years (mean age 37.9±7.5) completed the study, and data obtained from questionnaire [Table/Fig-2] showed that side effects such as gastric irritation, stomach bloating, stomach burning were reported for diclofenac 50 mg group in eight patients and no side effects were noted in low dose diclofenac group. [Table/Fig-4,5] show the mean and standard deviation of the results obtained using VAS, for Diclofenac 25 mg and Diclofenac 50 mg group respectively. A stastically significant lower pain score was observed in both groups, at all three days (p<0.05).
Showing the mean pain scores in the Low Dose Diclofenac 25 mg group obtained by using VAS.
| Diclofenac (25 mg) | N | Mean | Std. Deviation | Std. Error | 95% CI | Repeated measures ANOVA |
|---|
| Lower | Upper |
|---|
| Day 1 | 20 | 5.4 | 1.47 | 0.33 | 4.71 | 6.09 | F=105.7 p<0.005 |
| Day 2 | 20 | 4.35 | 1.39 | 0.31 | 3.70 | 5.00 |
| Day 3 | 20 | 0.75 | 0.72 | 0.16 | 0.42 | 1.09 |
*Significant (<0.05)
Showing the mean pain scores in the Diclofenac 50 mg group obtained by using VAS.
| Diclofenac (50 mg) | N | Mean | Std. Deviation | Std. Error | 95% CI | Repeated measures ANOVA |
|---|
| Lower | Upper |
|---|
| Day 1 | 20 | 5.4 | 1.79 | 0.40 | 4.56 | 6.24 | F=79.2 p<0.005 |
| Day 2 | 20 | 3.5 | 0.83 | 0.19 | 3.11 | 3.89 |
| Day 3 | 20 | 0.35 | 0.49 | 0.11 | 0.12 | 0.58 |
*Significant (<0.05)
[Table/Fig-6] shows comparison of pain scores in two study groups obtained by using VAS, when both the groups were compared with each other by using independent t-test. The results were statically non significant on day 1 vs day 2 (p=0.127), day 1 vs day 3(p=0.507) and day 2 vs day 3 (p=0.292).
Showing the mean of VAS scores in diclofenac 50 mg and low dose diclofenac 25 mg groups.
| Time period | Groups | N | Mean | SD | Mean Difference | Independent t test |
|---|
| T | p-value |
|---|
| Day 1 to Day 2 | Diclofenac (50 mg) | 20 | 1.05 | 1.15 | -0.85 | -1.56 | 0.127 |
| Diclofenac (25 mg) | 20 | 1.9 | 2.15 |
| Day 1 to Day 3 | Diclofenac (50 mg) | 20 | 4.65 | 1.69 | -0.4 | -0.67 | 0.507 |
| Diclofenac (25 mg) | 20 | 5.05 | 2.06 |
| Day 2 to Day 3 | Diclofenac (50 mg) | 20 | 3.6 | 0.99 | +0.45 | -1.069 | 0.292 |
| Diclofenac (25 mg) | 20 | 3.15 | 1.60 |
Discussion
Although the surgical extraction of impacted third molars is the most widely accepted model to compare the efficacy of analgesics and anti-inflammatory drugs, the prevention and control of pain after periodontal surgery is also of great concern for patients and clinicians. Several studies [25-29] have investigated different NSAIDs for this purpose, but to the authors’ knowledge, there was no available study on the use of low dose Diclofenac after periodontal surgery. This unprecedented clinical trial was designed to compare a combination agent (Diclofenac, 25 mg, and paracetamol, 325 mg) to the traditionally used analgesic (Diclofenac 50 mg, and paracetamol 325 mg) the authors have attempted to identify the possibility of using this low dose diclofenac 25 mg as an alternative and taking advantage of the fact that it has fewer side effects than diclofenac 50 mg.
To help physicians and patients to better comply with the recommendation of the use of the lowest effective doses of NSAIDs, a novel, nonselective NSAID was recently approved by the US Food and Drug Administration for use as a treatment for acute and chronic pain [22]. This NSAID, a lower-dose submicron version of diclofenac, was created using SoluMatrix Fine Particle Technology (IrokoPharmaceuticals, Philadelphia, PA) SoluMatrixdiclofenac capsules (35 mg) are equivalent to a 20% lower diclofenac dose compared with diclofenac potassium Immediate-Release (IR) 50 mg and have reported analgesia in clinical trials in patients experiencing acute pain after bunionectomy and pain associated with osteoarthritis [30]. In addition, SoluMatrixdiclofenac capsules were recently approved by the FDA for the management of mild to moderate acute pain and osteoarthritis-related pain [31].
Many studies used a VAS [32-35] or the VRS [36-38] for rating the patient’s pain perception during a 3-10-hour period of pain evaluation.
In this study, pain intensity was recorded for the 1st, 2nd and 3rd day after surgery; the pain scale was marked twice a day with a gap of eight hours in between. An eight hour period seems to be appropriate for pain intensity assessment because it covers well the duration of action of drug [39]. The pain threshold level after periodontal surgery is at its peak within the immediate 11-hour postoperative period. It is 25% to 40% same as that being reported after more extensive surgical interventions, such as wisdom teeth surgery and is greatest which explains why pain was rarely being reported on the following days [40,41]. In the present study, low-dose diclofenac 25 mg and diclofenac 50 mg reduced postoperative pain for the first eight hours, albeit with a declining effect. Considerable pain was reported after first eight hours in the low-dose diclofenac group by 9 (45%) patients compared to 20 (100%) patients. These results can be linked to the known pharmacokinetics of each drug. When diclofenac is taken postoperatively, the plasma concentration is expected to be below optimal levels at 5-6 hours after dosing [39]. There was no statistically significant (p>0.005) pain score difference between the two strengths when they were observed on day 1 vs day 3, day 1 vs day 2 and day 2 vs day 3 obtained by VAS scale [Table/Fig-6]. Both strengths when compared of low-dose diclofenac and diclofenac 50 mg, showed statistically significant lower pain scores (p<0.005) on all postoperative days [Table/Fig-4,5]. The early onset of action of the low-dose diclofenac 25 mg may be attributed to a new, proprietary dry milling process that creates submicron drug particles. This process improves absorption properties and enables efficacy at lower doses [42]. The average low-dose diclofenac 25 mg drug particle size achievable using this technology is 200 nm to 800 nm, approximately 10-20 times smaller than the traditionally available diclofenac 50 mg this increased surface area to mass ratio of the smaller drug particles ensuing in rapid dissolution [42]. In a phase 1 study, submicron diclofenac 35 mg (Zorvolexz) administration demonstrated a comparable time to reach peak plasma levels as diclofenac potassium immediate release 50 mg tablets, indicating a similar rate of absorption but with an overall systemic exposure that was 23% lower [16]. The results in the present study are in accordance with the results of previous studies in which low dose diclofenac 25 mg relieved the pain. Gibofsky A et al., performed a double-blind study in which the analgesic activities of low-dose diclofenac (35 mg Twice or Thrice daily) was evaluated, this enrolled patients >40 years of age with clinically and radiographically confirmed (Kellgren-Lawrence Grade II-III) hip or knee Osteoarthritis (OA). Submicron diclofenac 35 mg significantly improved WOMAC pain subscale scores from baseline at 12-weeks compared with placebo [30].
Gibofsky A et al., performed a study in which submicron diclofenac 18 mg and 35 mg three times daily demonstrated efficacy in a phase three study in patients with acute postoperative pain after bunionectomy surgery. Significant results were noted for these strengths and the preparations are approved in the United States for the treatment of mild to moderate acute pain in adults [43]. In the current study, statistically significant lower pain scores were observed in both groups. Diclofenac 25 mg was effectively able to reduce pain after periodontal flap surgery. This was the lowest possible dose that minimised gastric discomfort along with efficient analgesia. Therefore this low dose diclofenac 25 mg can be used as an alternative to traditional dose of 50 mg for the management of postoperative pain after periodontal flap surgery.
Limitation
As the study included VAS the pain measuring criteria is subjective. Pain threshold levels differs in every individual and it is gender subjective.
However, further studies need to be done on larger populations and on medically compromised patients, to provide a rational basis for the use of low-dose diclofenac effectively in postoperative pain relief.
Conclusion
The findings from the present study suggest that low dose diclofenac 25 mg can be effectively used as an alternative in reducing post-operative pain after periodontal flap surgery. It is more advantageous since the adverse effects exerted by low dose diclofenac 25 mg are minuscule as compared to the traditional dose of diclofenac 50 mg.
Further studies with larger sample size should be conducted to corroborate the effectiveness of low dose diclofenac.
*Significant (<0.05)
*Significant (<0.05)
[1]. Pihlstrom BL, Hargreaves KM, Bouwsma OJ, Myers WR, Goodale MB, Doyle MJ, Pain after periodontal scaling and root planingJ Am Dent Assoc 1999 130:801-07.10.14219/jada.archive.1999.030310377637 [Google Scholar] [CrossRef] [PubMed]
[2]. Curtis JW, Mclain JB, Hutchinson RA, The incidence and severity of complications and pain following periodontal surgeryJ Periodontol 1985 56:597-601.10.1902/jop.1985.56.10.5973863909 [Google Scholar] [CrossRef] [PubMed]
[3]. Scott DS, Hirshman R, Psychological aspects of dental anxiety in adultsJ Am Dent Assoc 1982 104:273110.14219/jada.archive.1982.01236948026 [Google Scholar] [CrossRef] [PubMed]
[4]. Vogel RI, Gross JI, The effects of nonsteroidal anti-inflammatory analgesics on pain after periodontal surgeryJ Am Dent Assoc 1984 109:731-34.10.14219/jada.archive.1984.01816333443 [Google Scholar] [CrossRef] [PubMed]
[5]. Cooper SA, Needle SE, Kruger GO, Comparative analgesic potency of aspirin and ibuprofenJ Oral Surg 1977 35:898-903. [Google Scholar]
[6]. Weiss B, Hait WN, Selective cyclic nucleotide phosphodiesterase inhibitors as potential therapeutic agentsAnnu Rev Pharmacol Toxicol 1977 17:441-47.10.1146/annurev.pa.17.040177.00230117360 [Google Scholar] [CrossRef] [PubMed]
[7]. Beaver WT, Impact of non-narcotic oral analgesics on pain managementAm J Med 1988 84:3-15.10.1016/0002-9343(88)90471-8 [Google Scholar] [CrossRef]
[8]. Food and Drug Administration Approval: (NDA) 019201 Voltaren (Diclofenac Sodium) 1988 [Google Scholar]
[9]. Bruno A, Tacconelli S, Patrignani P, Variability in the response to non- steroidal anti-inflammatory drugs: mechanisms and perspectivesBasic Clin Pharmacol Toxicol 2014 114:56-63.10.1111/bcpt.1211723953622 [Google Scholar] [CrossRef] [PubMed]
[10]. Patrono C, Patrignani P, Garcia Rodriguez LA, Cyclooxygenase- selective inhibition of prostanoid formation transducing biochemical selectivity into clinical read-outsJ Clin Invest 2001 108:7-13.10.1172/JCI20011341811435450 [Google Scholar] [CrossRef] [PubMed]
[11]. Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR, Nonsteroid drug selectivities for cyclo-oxygenase-1rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full invitro analysisProc Natl Acad Sci USA 1999 96:7563-68.10.1073/pnas.96.13.756310377455 [Google Scholar] [CrossRef] [PubMed]
[12]. Van Hecken A, Schwartz JI, Depre M, Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteersJ Clin Pharmacol 2000 40:1109-20. [Google Scholar]
[13]. Coxib And Traditional Nsaid Trialists’ (Cnt) CollaborationBhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Vascular and upper gastrointestinal effects of non steroidal anti-inflammatory drugs: meta analyses of individual participant data from randomised trialsLancet 2013 382:769-79.10.1016/S0140-6736(13)60900-9 [Google Scholar] [CrossRef]
[14]. Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase2 selective and non- selective non-steroidal anti-inflammatory drugs: nested case-control studyLancet 2005 365:475-81.10.1016/S0140-6736(05)17864-7 [Google Scholar] [CrossRef]
[15]. Hippisley-Cox J, Coupland C, Risk of myocardial infarction in patients taking cycloxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysisBMJ 2005 330:136610.1136/bmj.330.7504.136615947398 [Google Scholar] [CrossRef] [PubMed]
[16]. Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adultsCirculation 2004 109:2068-73.10.1161/01.CIR.0000127578.21885.3E15096449 [Google Scholar] [CrossRef] [PubMed]
[17]. Riera-Guardia N, Castellsague J, Calingaert B, The SOS Project: nonsteroidal anti-inflammatory drugs and upper gastrointestinal complications. Meta-analysis of epidemiological studiesPresented at: 26th International Conference on Pharmacoepidemiology and Therapeutic Risk Management 2010 :19-22. [Google Scholar]
[18]. McGettigan P, Henry D, Cardiovascular risk with non- steroidal anti-inflammatory drugs: systematic review of population based controlled observational studiesPLoS Med 2011 8:e100109810.1371/journal.pmed.100109821980265 [Google Scholar] [CrossRef] [PubMed]
[19]. Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (theSOSProject)Drug Saf 2012 35:1127-1146.10.1007/BF0326199923137151 [Google Scholar] [CrossRef] [PubMed]
[20]. Schneider V, Lévesque LE, Zhang B, Hutchinson T, Brophy JM, Association of selective and conventional non-steroidal anti-inflammatory drugs with acute renal failure: a population-based, nested case-control analysisAm J Epidemiol 2006 164:881-89.10.1093/aje/kwj33117005625 [Google Scholar] [CrossRef] [PubMed]
[21]. Hernández-Díaz S, Rodríguez LA, Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding /perforation: an overview of epidemiologic studies published in the1990sArch Intern Med 2000 160:2093-99.10.1001/archinte.160.14.209310904451 [Google Scholar] [CrossRef] [PubMed]
[22]. Food and Drug Administration, US Dept of Health and Human Services. Public health advisory: FDA announces important changes and additional warnings for COX-2 selective and non- selective non-steroidal anti- inflammatory drugs (NSAIDs). 2005 [Google Scholar]
[23]. European Medicines Agency. Opinion of the Committee for Medicinal Products for Human Use pursuant to Article-5(3) of Regulation (EC), No726/2004, for non-selective non-steroidal anti-inflammatory drugs (NSAIDs). 2006 [Google Scholar]
[24]. Health Canada. Basic product monograph information for non- steroidal anti-inflammatory drugs (NSAIDs). 2006 [Google Scholar]
[25]. Vogel RI, Desjardins PJ, Major KVO, Comparison of presurgical and immediate postsurgical ibuprofen on postoperative periodontal painJ Periodontol 1992 63:914-18.10.1902/jop.1992.63.11.9141453306 [Google Scholar] [CrossRef] [PubMed]
[26]. Trombelli L, Schincaglia P, Zangari F, Scapoli C, Calura G, Effect of pretreatment with ketorolac tromethamine on postoperative pain following periodontal surgeryJ Clin Periodontol 1996 23:128-32.10.1111/j.1600-051X.1996.tb00545.x8849849 [Google Scholar] [CrossRef] [PubMed]
[27]. Tucker PW, Smith JR, Adams DF, A comparison of two analgesic regimens for the control of postoperative periodontal discomfortJ Periodontol 1996 67:125-29.10.1902/jop.1996.67.2.1258667132 [Google Scholar] [CrossRef] [PubMed]
[28]. Gallardo F, Rossi E, Analgesic efficacy of flurbiprofen as compared to acetaminophen and placebo after periodontal surgeryJ Periodontol 1990 61:224-27.10.1902/jop.1990.61.4.2242324921 [Google Scholar] [CrossRef] [PubMed]
[29]. Gallardo F, Rossi E, Effects of sodium meclofenamate on postoperative pain following periodontal surgeryJ Periodontol 1992 63:166-68.10.1902/jop.1992.63.3.1661593410 [Google Scholar] [CrossRef] [PubMed]
[30]. Gibofsky A, Hochberg MC, Jaros M, Young CL, Efficacy and safety of low-dose submicron diclofenac for the treatment of osteoarthritis pain: a 12 week, phase 3 studyCurr Med Res Opin 2014 30:1883-93.10.1185/03007995.2014.94612325050589 [Google Scholar] [CrossRef] [PubMed]
[31]. Zorvolex highlights of prescribing information. published 2013. Available at https://www.iroko.com/wp-contents/uploads/2013/10/zorvolex [Google Scholar]
[32]. Betancourt JW, Kupp LI, Jasper SJ, Farooki OW, Efficacy of ibuprofen-hydrocodone for the treatment of postoperative pain after periodontal surgeryJ Periodontol 2004 75:872-86.10.1902/jop.2004.75.6.87215295955 [Google Scholar] [CrossRef] [PubMed]
[33]. Cooper S, Five studies on ibuprofen for postsurgical dental painAm J Med 1984 70:70-77.10.1016/S0002-9343(84)80022-4 [Google Scholar] [CrossRef]
[34]. Ong CK, Seymour RA, Lirk P, Merry AF, Combining paracetamol (acetaminophen) with nonsteroidal anti inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative painAnesth Analg 2010 110:1170-79.10.1213/ANE.0b013e3181cf928120142348 [Google Scholar] [CrossRef] [PubMed]
[35]. Wuolijoki E, Oikarinen VJ, Ylipaavalniemi P, Hampf G, Tolvanen M, Effective postoperative pain control by preoperative injection of diclofenacEur J Clin Pharmacol 1987 32:249-52.10.1007/BF006075713595697 [Google Scholar] [CrossRef] [PubMed]
[36]. Konuganti K, Rangaraj M, Elizabeth A, Pre-emptive 8 mg dexamethasone and 120 mg etoricoxib for pain prevention after periodontal surgery: A randomized controlled clinical trialJ Indian Soc Periodontol 2015 19(4):474-76.10.4103/0972-124X.15347526392703 [Google Scholar] [CrossRef] [PubMed]
[37]. Rashwan WA, The efficacy of acetaminophen-caffeine compared to Ibuprofen in the control of postoperative pain after periodontal surgery: a crossover pilot studyJ Periodontol 2009 80:945-52.10.1902/jop.2009.08063719485825 [Google Scholar] [CrossRef] [PubMed]
[38]. Schirmer C, Dos Santos GO, Rost JF, Ferreira MBC, Weidlich P, Factors associated with pain and analgesic consumption following non-surgical periodontal therapy under local anaesthesia and carried out by dental studentsJ Clin Periodontol 2018 45:68-77.10.1111/jcpe.1283329078012 [Google Scholar] [CrossRef] [PubMed]
[39]. Roberts LJ, Morrow JD, Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, edsGoodman and Gilman’s The Pharmacological Basis of Therapeutics 2001 10th edNew YorkMcGraw-Hill, Medical Publication Division:703 [Google Scholar]
[40]. Jorkjend L, Skoglund A, Effect of non-eugenol and eugenol-containing periodontal dressing on the incidence and severity of pain after periodontal soft tissue surgeryJ Clin Periodontol 1990 17:341-44.10.1111/j.1600-051X.1990.tb00028.x2398130 [Google Scholar] [CrossRef] [PubMed]
[41]. Skoglund LA, A new paracetamol/paracetamol methionine ester combination. Effects on postoperative courseEur J ClinPharmacol 1986 31:45-48.10.1007/BF008709843780826 [Google Scholar] [CrossRef] [PubMed]
[42]. Voltron XR (diclofenac sodium extended release); US prescription. East Hanover, NJ; Novalis pharmaceuticals corporations, 2011. Available at: http://www.Pharma.us.novartis.com/product/pi/voltren_xr.pdf [Google Scholar]
[43]. Gibofsky A, Silberstein S, Argoff C, Daniels S, Jensen S, Young CL, Lower-dose diclofenac submicron particle capsules provide early and sustained acute patient pain relief in a phase 3 studyPostgrad Med 2013 125:130-38.10.3810/pgm.2013.09.269324113671 [Google Scholar] [CrossRef] [PubMed]