Bone is a dynamic tissue that grows in size during childhood and accelerates its growth during adolescence. The peak bone mass is attained at the age of 35 years, after which it starts declining with increase in age. The normal bone density is defined as: 1 Standard Deviation (SD) or greater than the mean value in young adults of the same age and sex (t-score of – 2.5). Bone density < 2.5 SD of peak bone mass and impaired bone quality is called osteoporosis [1].
Osteoporosis is a metabolic bone disease which involves reduction in bone mass and deterioration of the bone tissue micro architecture leading to enhanced bone fragility [2]. Initially, osteoporosis is asymptomatic and noticed only after a fracture. It is globally considered as a silent killer due to its propensity to cause fracture in the elderly age group. Primary osteoporosis is commonly seen in women after about 10 years of the cessation of ovarian function. The general prevalence of primary osteoporosis in India has been reported to be 42.5% among women aged 50 years and above [3]. It is also estimated that every 1 out of 5 males and 1 out of 3 females suffer from osteoporosis, making India one of the largest affected countries in the world [4].
The gold standard for diagnosing future osteoporotic hip and spine fractures are BMD and DEXA, which is unaffordable, and compression fractures of the vertebrae are difficult to detect as these are asymptomatic. As a result, the BTMs have recently come to trend in assessing the fracture risks. BTMs are protein derivatives released during bone remodelling [5]. The bone formation marker OC is synthesised predominantly by the osteoblasts and incorporated into the extracellular matrix of bone. Osteocalcin is dependent on post translational carboxylation for its hydroxyapatite affinity. Bone remodelling is a continuous process, involving both bone formation and bone resorption, which in turn relies on the activities of osteoclasts (resorption), osteoblasts (formation) and osteocytes (maintenance).
In healthy adults, bone formation is coupled with bone resorption, wherein the rate of resorption is equal to the rate of formation which is regulated by various hormones and local mediators. An imbalance in bone turnover is seen especially in metabolic bone diseases such as osteoporosis, somatic growth, ageing and other conditions affecting bone turnover. This imbalance is primarily due to uncoupling of bone resorption and formation, where both processes may not occur efficiently at the same time. Therefore, osteocalcin serves as a valid marker to detect dynamic changes in bone turnover, both when formation and resorption are coupled as well as uncoupled [6,7].
The traditional resorption marker measures the collagen degradation products from osteoclasts. Hydroxyproline is derived from proline that undergoes post-translational hydroxylation to form collagen, a major component of the bone. The hydroxylysine glycosides are integral parts of bone collagen and occur in two forms: glycosyl-galactosyl hydroxylysine (Glc-Gal-Hyl) and galactosyl-hydroxylysine (Gal-Hyl). Both components are released into the circulation during collagen degradation and can be measured in urine [8].
The aim of the present study was to assess the difference in the levels of BTMs in various menstrual stages of women and its applicability as a diagnostic tool for primary osteoporosis in order to substantially reduce the morbidity and mortality in the study population.
Materials and Methods
The cross-sectional study was conducted on 105 women visiting the Department of Orthopaedics and Gynaecology at AJ Institute of Medical Science & Research Centre, Mangalore from July 2011–December 2012, after approval from the Institution Ethical Committee (AJEC/2009/34). The current mean and SD obtained for serum osteocalcin among three groups (35 subjects in each group) was applied in G*power (statistical software), which yielded a minimum sample size of 15 (5 in each group). Alpha error being 5%, Power being 95% and effect size 1.25. The subjects were divided into three groups which included:
Group I: consisting of 35 pre-menopausal women.
Group II: consisting of 35 post-menopausal women without fractures.
Group III: consisting of 35 post-menopausal women with fractures (sample was collected on the day of the fracture) Fractures were sustained as a result of trivial trauma involving the wrist, spine and hip.
Exclusion criteria involved subjects with chronic illness, kidney and liver disorder, diabetes, surgically induced menopause, patients on hormonal therapy, steroidal therapy, subjects having fractures as a result of road traffic accident and pregnant women.
An informed consent was obtained from all the study participants. 5 mL of venous blood sample was collected in a plain vacutainer and centrifuged at 4000 rpm. Serum was then analysed for OC, alkaline phosphatase (ALP), calcium, phosphorous, total protein and albumin. A 24-hour urine sample was also collected and taken after adding a preservative (HCl: 5 mL/L of urine) to analyse urinary hydroxyproline.
Methods of Estimation
Estimation of serum calcium was done by Arsenazo method [9], inorganic phosphorous by phosphomolybdate method [10], ALP by International Federation of Clinical Chemistry (IFCC)/kinetic method [11], total protein by biuret method [12] and albumin by bromocresol green method [13] using Agappe reagent kits on LabLife RoboChem autoanalyser. Serum OC was estimated by chemiluminescent method [14] in autoanalyser Siemens’ Immulite 1000 and urinary hydroxyproline by modified Neuman and Logan method [15] in a spectrophotometer using commercially available kits.
Statistical Analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) version 16.0 Categorical variables were represented as frequency and percentage. Continuous variables were represented as mean±SD. ANOVA test was done to compare variables among three groups. Intergroup comparison was done using Tukey’s test. Comparison study between the variables of two groups was done using student’s t-test. Karl Pearson’s test correlation was used to correlate the data. Statistical significance was considered as p < 0.05.
Results
The study group consisted of 35 pre-menopausal, 35 post-menopausal and 35 osteoporotic post-menopausal women. The age group of the study participants ranged from 20 to 80 years with the mean age (in years) being 28.37±5.28, 57.09±7.16 and 68.34±8.13 in group I, II and III respectively.
The mean BMI was 18.68±2.40 kg/m2 in osteoporotic post-menopausal women as compared to the post-menopausal women (22.32±2.28 kg/m2) and pre-menopausal women (18.68±2.40 kg/m2). This difference was statistically very highly significant (p<0.001). A significant decrease was observed in group III to group I and group III to group II (p<0.05).
The mean serum OC levels in group III, II and I were 2.79±2.33, 12.61±4.34 and 5.68±2.83 ng/mL respectively, which was statistically significant (p-value <0.001). OC levels had significantly decreased from group II to group I, group II to group III and group I to group III (p<0.05).
Mean urinary hydroxyproline was statistically higher in group III (27.88±6.67 mg/dL) when compared to the other two groups (p<0.001). It was interesting to note a statistically higher mean serum ALP in post-menopausal women with fractures (105±44.36 U/L) when compared to the other two groups. The mean serum calcium level was lowest in group III (8.69±0.64 mg/dL) (p<0.001) [Table/Fig-1].
Comparison of clinical and biochemical parameters between the study groups.
| Parameter | Group I (n=35) | Group II (n=35) | Group III (n=35) |
|---|
| Age (years) | 28.37±5.28 | 57.09±7.16 a* | 68.34±8.13 b*c* |
| BMI (kg/m2) | 21.50±2.03 | 22.32±2.28 | 18.68±2.40 b*c* |
| Age of attaining menarche (years) | 12.71±0.57 | 12.40±1.04 | 12.57±0.98 |
| Age of attaining menopause (years) | - | 48.74±2.72 | 48.26±2.85 |
| Time since menopause (years)† | - | 8.34±6.27 | 20.09±8.38 |
| S. Calcium (mg/dL) | 9.32±0.64 | 9.17±0.65 | 8.69±0.64 b*c* |
| S. Phosphate (mg/dL) | 2.79±0.57 | 2.78±0.55 | 2.95±0.86 |
| S. ALP (U/L) | 75.00±10.06 | 95.75±20.63 a* | 105.26±44.36 b* |
| S. Total Protein (g/dL) | 6.53±0.41 | 6.51±0.32 | 6.31±0.56 |
| S. Albumin (g/dL) | 3.99±0.23 | 4.03±0.18 | 3.65±0.38 b*c* |
| S. Osteocalcin (ng/mL) | 5.68±2.83 | 12.61±4.34 a* | 2.79±2.33 b*c* |
| Urinary Hydroxyproline (mg/dL) | 5.73±2.53 | 16.66±3.32 a* | 27.88±6.67 b*c* |
BMI: Body Mass Index; ALP: Alkaline Phosphatase; a: comparison between Group I and Group II; b: comparison between Group I and Group III; c: comparison between Group II and Group III;
*: p <0.05; level of significance obtained from Tukey’s test (post-hoc analysis/intergroup comparison); †: Age minus Menopause Age
Correlation studies considering all three groups revealed a highly significant (p<0.001) positive correlation of serum OC with BMI (r=0.484) and albumin (r=0.379). Urinary hydroxyproline exhibited a statistically significant strong positive correlation with age (r=0.806, p<0.001) and an inverse correlation with BMI (r= -0.441) and calcium (r=-0.341) (p<0.001) [Table/Fig-2].
Correlation of bone markers with various parameters in all study subjects (n=105).
| Pearson’s correlation (r) |
|---|
| Variables | Age | BMI | Serum Calcium | Serum Phosphorus | Serum ALP | Serum Total Protein | Serum Albumin | Serum Osteocalcin | Urinary Hydroxyproline |
|---|
| Serum Osteocalcin | -0.080 | 0.484** | 0.184 | -0.106 | -0.031 | 0.089 | 0.379** | 1.000 | -0.139 |
| Urinary Hydroxyproline | 0.806** | -0.441** | -0.341** | 0.182 | 0.479** | -0.194* | -0.392** | -0.139 | 1.000 |
BMI: Body Mass Index; ALP: Alkaline Phosphatase; * p <0.05, level of significance obtained from Pearson’s correlation test; ** p <0.001, level of significance obtained from Pearson’s correlation test
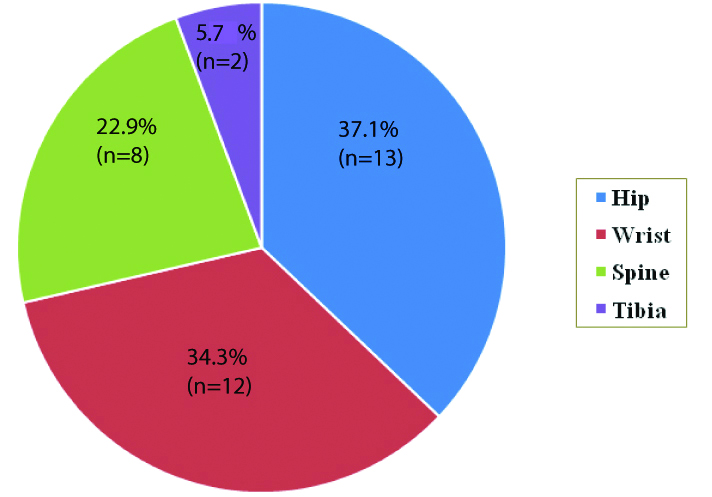
Group III was further divided according to the fracture types in which 37.1% (n=13) had hip fractures, 34.3% (n=12) wrist fractures, 22.9% (n=8) spine fracture and 5.7% (n=2) tibia fracture [Table/Fig-3]. The significance of BTMs were analysed on the different fracture types except tibia due to a very small number. Overall, serum OC was higher among subjects with wrist fracture (2.44±0.34 ng/mL). A statistically high significant difference was present in the mean serum osteocalcin values between the wrist fractures and spine fractures (p<0.05), although the urinary hydroxyproline level was not significantly different in the various fracture groups [Table/Fig-4].
Distribution of fracture types among post-menopausal women with fracture (Group III).
Biochemical parameters based on various fracture types in post-menopausal women with fracture.
| Parameter | Hip Fracture (n=13) | Wrist Fracture (n=12) | Spine Fracture (n=8) |
|---|
| S. Calcium (mg/dL) | 8.48±0.65 | 8.63±0.49 | 9.13±0.78 |
| S. Phosphorus (mg/dL) | 2.78±0.94 | 2.96±0.87 | 3.25±0.84 |
| S. ALP (U/L) | 119.46±61.23 | 87.17±20.36 | 117.13±33.23 |
| S. Total Protein (g/dL) | 6.09±0.62 | 6.50±0.33 | 6.55±0.57 |
| S.Albumin (g/dL) | 3.43±0.42 | 3.77±0.23 | 3.84±0.42a* |
| S. Osteocalcin (ng/mL) | 2.19±0.31 | 2.44±0.34 | 2.01±0.02b* |
| Urinary hydroxyproline (mg/dL) | 27.29±6.89 | 26.67±5.54 | 32.16±7.23 |
ALP: Alkaline Phosphatase; a: comparison between spine fracture and hip fracture; b: comparison between spine fracture and wrist fracture; *: p <0.05, level of significance obtained from Tukey’s test (post-hoc analysis/ intergroup comparison)
Discussion
Detection of osteoporosis in post-menopausal women at an early stage, before development of bone fractures is essential. Hence, the efficacy of BTMs on the entire bone and its effect on various factors causing osteoporosis needs to be extensively studied. In this study, we observed very low levels of serum OC and high levels of urinary hydroxyproline excretion in osteoporotic post-menopausal women as compared to the women in other two groups. A study conducted in Sweden, also shows 20% lower OC levels among the post-menopausal women with fractures than healthy post-menopausal women. Similar findings were also seen in a study by Pietschmann P et al., [16]. However, the present study findings were not in accordance with the study carried out by Kalaiselvi VS et al., which reported a high level of OC in osteoporotic post-menopausal women compared to non osteoporotic women [17].
Present study showed increased urinary hydroxyproline excretion in osteoporotic women in comparison to post-menopausal women without fractures, which was similar to the study findings of Jagtap VR et al., [18] and a pilot study done by Rai T et al., [19].
Pearson correlation coefficient showed a positive correlation of urinary hydroxyproline and negative correlation of OC to age in this study which is in parallel to a study done by Das M et al., [20]. In late menopause, bone formation is reduced and resorption activity is increased which is reflected by decrease in OC levels and increase in urinary excretion of hydroxyproline, which indicates the degradation of type I collagen of bone matrix thus explaining the propensity to fractures in these women even with a trivial fall. At the molecular level, it can be explained on the basis of deficiency of oestrogen, which accelerates the receptor activator of nuclear factor kappa B ligand (RANKL) that decreases osteoporosis [20]. However, hydroxyproline is found in other tissues and can be liberated in their abnormalities. In early menopause, there is an accelerated osteoclastic activity due to the sudden depletion of oestrogen. This increases the bone resorption and as bone re-modelling is coupled, serum OC and urinary hydroxyproline increases.
Decreased BMI is directly proportional to low BMD causing osteoporotic fractures in older women as shown in this study where osteoporotic post-menopausal women had significantly low mean BMI as compared to the women in the other two groups and had a positive significant correlation with serum OC. This is supported by a study done by Morin S et al., which concluded that low bone mass, body weight or visceral fat and low OC can predict osteoporosis, making serum OC a highly sensitive bone formation marker [21]. Sri Rekha P et al., similarly showed a significant negative correlation of urinary hydroxyproline and BMI [22] and inverse results was seen in a study by Paneri S et al., [23].
Exhaustion of oestrogen in menopause has deleterious effects on the health of menopausal women and one of these is due to increased excretion of calcium and decreased absorption in the intestine. In the present study, we observed low mean serum calcium level in osteoporotic women as compared to the other groups and serum calcium showed a positive correlation with serum OC. A significant negative correlation was seen with urinary hydroxyproline which was in parallel to the results obtained by Prabha YS et al., [24].
Serum ALP is a bone formation marker and is present in various other tissues. In our study, it showed a negative correlation with serum OC which has the same action and a significant positive correlation with urinary hydroxyproline. Increase in serum ALP may also occur in osteoporotic women due to intake of certain drugs affecting liver enzymes or systemic response of the body to injury following a fracture. Therefore, serum ALP is a non-specific bone formation marker. These findings were consistent with a study by Bowles SA et al., [25].
Albumin is a major component of protein and a sensitive marker of poor nutritional status. This study showed statistically lower serum albumin levels in osteoporotic post-menopausal group and showed a positive correlation with OC. Similarly a longer duration of lower serum albumin levels was observed in a few studies, which made the patient susceptible to fractures [26]. The mechanism of hypo-albuminemia in osteoporosis remains controversial. It could be either due to albumin being present in the osteoid or it may directly cause changes in bone remodelling with release of NF-κb which activates osteoclasts and decreases osteoblasts. Hence, explaining its positive significant correlation with serum OC and negative significant correlation with urinary hydroxyproline.
Serum OC was statistically lower in vertebral fractures as compared to wrist fractures and no statistical difference was seen among the spine and hip fractures which is in accordance with a study done by Soroush M et al., who showed that the mean urinary hydroxyproline levels had no statistical difference in the three types of fracture [27].
Limitation
A small sample size restrained authors from finding the exact cut-off value of the BTMs in each fracture type and the effect of BTMs on different osteoporotic treatment modalities could have been evaluated.
Conclusion
Bone turnover markers can be an inexpensive diagnostic and screening tool for primary osteoporosis. Low serum OC levels and high urinary hydroxyproline levels in post-menopausal women could thus predict osteoporosis and its timely medical intervention could prevent the risk of osteoporotic fractures thereby decreasing the morbidity and mortality.
BMI: Body Mass Index; ALP: Alkaline Phosphatase; a: comparison between Group I and Group II; b: comparison between Group I and Group III; c: comparison between Group II and Group III;
*: p <0.05; level of significance obtained from Tukey’s test (post-hoc analysis/intergroup comparison); †: Age minus Menopause Age
BMI: Body Mass Index; ALP: Alkaline Phosphatase; * p <0.05, level of significance obtained from Pearson’s correlation test; ** p <0.001, level of significance obtained from Pearson’s correlation test
ALP: Alkaline Phosphatase; a: comparison between spine fracture and hip fracture; b: comparison between spine fracture and wrist fracture; *: p <0.05, level of significance obtained from Tukey’s test (post-hoc analysis/ intergroup comparison)