Now-a-days, it is well-known that intrauterine life has a direct role in the development of chronic diseases in adulthood. This concept states that early life conditions lead to foetal programming, which in turn results in adverse health outcomes later on [1]. Foetal development depends mainly on maternal nutrition and fetoplacental circulation [2]. Pregnancy is always associated with increased demand of all nutrients. The importance of mineral balance during this period of life is still underestimated; only little research documented the vital role of trace elements and minerals for the development of foetus [3]. It was noted that many trace elements in the foetal stores are transferred only in the last trimester of pregnancy [4]. In other words, foetal growth is a result of the interaction between its genetic potential and the intrauterine environment. Mothers who enter pregnancy in good health and who didn’t suffer any chronic illness or nutritional deprivation in childhood will get larger and healthier infants than mothers who didn’t have such advantages. Neonatal period is highly vulnerable period during which many psychological, physiological adjustments to the extra-uterine life must be made [5].
Zinc (Zn) is considered as one of the essential trace elements for proper DNA synthesis. It is also important for healthy immune system as it is needed for activation of T lymphocytes which attack infected cells [6].
Iron (Fe) in all cells is essential for erythrocyte oxygen transport and oxidative metabolism as it functions as a catalyst. Iron is present in erythrocytes (70%) and 30% in storage and a small amount in myoglobin and cytochromes [7]. The iron content of the newborn infant is a main source of haemoglobin formation in the first few months of life [8].
Serum iron and TIBC measurements are applied as screening tests for iron-deficiency anemia and chronic inflammatory disorders [9].
It has been confirmed that various trace elements such as Fe, P, Mg, Zn, Ca, Cu, etc., are metabolically interrelated with alterations in their levels during pregnancy that affects the birth outcome. However, there are multiple aspects regarding their impact, which still need further elucidation [10].
Until now studies have focused on evaluating the effects of a small number of trace elements on foetal growth and birth outcome [5]. During Pregnancy, the deficiency of the essential nutrients could affect pregnancy, delivery and outcome of pregnancy.
The purpose of the current study was to assess the relationship between maternal and neonatal cord blood levels of zinc, iron and TIBC and how the maternal status could be a screening approach for neonatal iron deficiency anemia.
Materials and Methods
This cross sectional, pilot study was carried out in the Labour ward, Cairo University Hospital on the mothers and their neonates (n=51 paired samples) of low socioeconomic status, who reported to the hospital between February 2018 and May 2018. Healthy mothers without diseases such as hypertension, diabetes, infections were included in the study. All mothers, when it was indicated, received a full course of steroids before delivery. The present study didn’t include mothers with multiple gestations. Neonates with chromosomal abnormalities and/or congenital malformations and mothers on Iron or Zinc supplements were excluded from the study.
The present study was approved by the Medical Research Ethical Committee of National Research Centre. Written informed consent from both parents were taken after full explanation of the study protocol.
Each mother was asked full medical history laying stress on gestational age, mode of delivery and birth order. Anthropometric evaluation including weight, height and body mass index was done. Laboratory investigations for serum levels of zinc, iron and TIBC were quantified.
Each neonate was subjected to thorough clinical examination with APGAR score calculated. Anthropometric evaluation including weight, height and head circumference was done. Laboratory Investigations for cord blood levels of zinc, iron and TIBC were done.
Methods
Paired maternal and neonatal cord blood samples were collected from the mother’s ante-cubital vein and from the placental end of the umbilical cord without milking.
Determination of Iron
Serum total iron was estimated by colorimetric method using kit purchased from Salucea Company (Netherlands) according to the method described by Stookey LL [11]. This method depends on the reaction of iron with chromazurol B and cetyltrimethyl-ammonium bromide to form a coloured complex where the intensity of the color is directly proportional to the concentration of iron in the sample.
Determination of Direct Serum TIBC
Serum TIBC was determined colorimetrically by using the kit obtained from Salucea Company (Netherlands) according to the method of Stookey LL [11]. TIBC represents the highest ratio of iron that can bound by serum protein. TIBC is highly correlated with serum transferrin (the primary serum iron transport protein) where more than 95% of serum non-heme iron is bound by transferrin. Only 30% of the available serum iron-binding sites are occupied, and changes in the ratio of serum iron to TIBC indicate changes in the body iron stores.
Determination of Zinc
Serum Zn was evaluated by colorimetric method using kit provided by Salucea Company (Netherlands) according to the method of Johnsen O et al., [12]. This method is based on the formation of a red chelate complex between Zn and 2-(5-Brom-2-pyridylazo)-5-(N-propyl-N-sulfopropylamino)-phenol. The elevation in the optical density can be estimated and is directly proportional to the concentration of total zinc in the serum.
Statistical Analysis
The collected data were coded, tabulated and statistically analysed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013.
Descriptive statistics were done for quantitative data as minimum and maximum of the range as well as mean±SD (standard deviation) for quantitative normally distributed data, while it was done for qualitative data as number and percentage.
Inferential analyses were done for quantitative variables, ANOVA test for more than two independent groups with normally distributed data. In qualitative data, inferential analyses for independent variables were done using Fisher-Exact test for differences between proportions with small expected numbers. While correlations were done using Pearson correlation for numerical normally distributed data. Linear regression model was used to find out independent factors affecting certain conditions. The level of significance was taken at p-value <0.05 (significant), otherwise non-significant.
Results
A total number of 51 mothers with their neonates participated in the present study. Mean age of mothers was 26.5±5.7 years, BMI mean was 27.8±3.7 and BMI grade recorded that 23.5% of the total number of mothers were normal, 45.1% were overweight and 31.4% were obese. Mothers who ate breakfast daily before pregnancy were 78.4% while who ate breakfast daily during pregnancy was 76.5% [Table/Fig-1].
Demographic characteristics of the studied mothers.
| Variables | Mean±SD | Range |
|---|
| Age (years) | 26.5±5.7 | 17.0-38.0 |
| BMI (kg/m2) | 27.8±3.7 | 19.5-36.3 |
| N | % |
| BMI grade | Normal | 12 | 23.5 |
| Over weight | 23 | 45.1 |
| Obese | 16 | 31.4 |
| Breakfast daily before pregnancy | 40 | 78.4 |
| Breakfast daily during pregnancy | 39 | 76.5 |
| Three meals daily | 33 | 64.7 |
| Meat in lunch | 1-2/week | 22 | 43.1 |
| 3/week | 23 | 45.1 |
| >3/week | 6 | 11.8 |
| Eating Vegetables | 1-2/week | 12 | 23.5 |
| 3/week | 20 | 39.2 |
| >3/week | 19 | 37.3 |
| Eating fruits | 1-2/week | 9 | 17.6 |
| 3/week | 22 | 43.1 |
| >3/week | 20 | 39.2 |
| Dairy products | 1-2/week | 12 | 23.5 |
| 3/week | 15 | 29.4 |
| >3/week | 24 | 47.1 |
Total=51
Neonatal mean gestational age was 36.6±2.5 weeks, weight grade recorded that 88.2% of the total number of neonates were normal, 7.8% were overweight and 3.9% were underweight and mode of delivery was cesarean section (54.9%) while 45.1% was normal vaginal delivery [Table/Fig-2].
Demographic characteristics of the studied neonates.
| Variables | Mean±SD | Range |
|---|
| Gestational age (weeks) | 36.6±2.5 | 28.0-41.0 |
| Weight z-score | 0.05±1.31 | -3.51-2.84 |
| APGAR score | 8.6±0.6 | 7.0-10.0 |
| Birth order | 2.4±1.2 | 1.0-5.0 |
| N | % |
| Sex | Male | 24 | 47.1 |
| Female | 27 | 52.9 |
| Weight grade | Low | 2 | 3.9 |
| Normal | 45 | 88.2 |
| High | 4 | 7.8 |
| Mode of delivery | Normal Vaginal Delivery | 23 | 45.1 |
| Cesarean Section | 28 | 54.9 |
Total=51
Serum zinc level was low in 100% of the studied mothers while serum iron was high (70.6%) in the majority of them and serum TIBC was high in 45.1% [Table/Fig-3].
Laboratory findings of the studied mothers.
| Variables | Mean±SD | Range |
|---|
| Serum zinc (μg/dL) | 19.2±9.2 | 7.3-39.2 |
| Serum iron (μg/dL) | 206.1±74.1 | 32.0-318.5 |
| Serum TIBC (μg/dL) | 539.5±439.8 | 58.8-1883.3 |
| N | % |
| Serum zinc | Low | 51 | 100.0 |
| Serum iron | Low | 1 | 2.0 |
| Normal | 14 | 27.5 |
| High | 36 | 70.6 |
| Serum TIBC | Low | 11 | 21.6 |
| Normal | 17 | 33.3 |
| High | 23 | 45.1 |
Total=51
Serum zinc level was low in 98% of the studied neonates, serum iron was normal in 76.5% while serum TIBC was high in 70.6% [Table/Fig-4].
Laboratory findings of the studied neonates.
| Variables | Mean±SD | Range |
|---|
| Serum zinc (μg/dL) | 22.6±12.4 | 7.3-57.0 |
| Serum iron (μg/dL) | 166.3±53.5 | 90.1-300.7 |
| Serum TIBC (μg/dL) | 452.9±259.6 | 101.0-1066.6 |
| N | % |
| Serum zinc | Low | 50 | 98.0 |
| Normal | 1 | 2.0 |
| Serum iron | Low | 8 | 15.7 |
| Normal | 39 | 76.5 |
| High | 4 | 7.8 |
| Serum TIBC | Low | 7 | 13.7 |
| Normal | 8 | 15.7 |
| High | 36 | 70.6 |
Total=51
Serum iron was significantly highest in neonates with high serum iron, followed by normal and least in low [Table/Fig-5].
Comparison between grades of neonatal iron regarding maternal laboratory findings.
| Variables | Low (N=8) | Normal (N=39) | High (N=4) | p |
|---|
| Serum zinc (μg/dL) | 16.2±3.2 | 20.3±9.7 | 14.6±11.0 | ^0.304 |
| Serum iron (μg/dL) | 160.1±69.9 | 208.7±71.3 | 272.9±64.4 | ^0.038* |
| Serum TIBC (μg/dL) | 528.8±360.1 | 565.5±467.7 | 307.2±263.2 | ^0.543 |
| Serum iron | Low | 1 (12.5%) | 0 (0.0%) | 0 (0.0%) | #0.284 |
| Normal | 3 (37.5%) | 10 (25.6%) | 1 (25.0%) |
| High | 4 (50.0%) | 29 (74.4%) | 3 (75.0%) |
| Serum TIBC | Low | 2 (25.0%) | 7 (17.9%) | 2 (50.0%) | #0.441 |
| Normal | 2 (25.0%) | 15 (38.5%) | 0 (0.0%) |
| High | 4 (50.0%) | 17 (43.6%) | 2 (50.0%) |
^Independent t-test, #Fisher-Exact test, *Significant
Gestational age was significantly highest in neonates with high serum iron, followed by normal and least in low. Birth order was significantly lowest in neonates with high serum iron, followed by normal and least in low as shown in [Table/Fig-6].
Comparison between grades of neonatal iron regarding neonatal demographic characteristics.
| Variables | Low (N=8) | Normal (N=39) | High (N=4) | p |
|---|
| Gestational age (weeks) | 34.5±2.9 | 36.8±2.2 | 38.8±2.2 | ^0.010* |
| Weight z-score | 0.83±1.51 | -0.03±1.21 | -0.73±1.53 | ^0.112 |
| APGAR score | 8.4±0.7 | 8.6±0.6 | 9.0±0.0 | ^0.265 |
| Birth order | 3.1±1.1 | 2.4±1.2 | 1.3±0.5 | ^0.038* |
| Sex | Male | 3 (37.5%) | 19 (48.7%) | 2 (50.0%) | #0.886 |
| Female | 5 (62.5%) | 20 (51.3%) | 2 (50.0%) |
| Weight grade | Low | 0 (0.0%) | 1 (2.6%) | 1 (25.0%) | #0.125 |
| Normal | 6 (75.0%) | 36 (92.3%) | 3 (75.0%) |
| OW | 2 (25.0%) | 2 (5.1%) | 0 (0.0%) |
| Mode of delivery | NVD | 5 (62.5%) | 15 (38.5%) | 3 (75.0%) | #0.250 |
| CS | 3 (37.5%) | 24 (61.5%) | 1 (25.0%) |
^Independent t-test, #Fisher-Exact test, *Significant, OW-Over Weight, NVD-Normal Vaginal Delivery, CS-Cesarean Section
Significant positive correlation between maternal and neonatal zinc has been recorded (r=0.322, p=0.021), as shown in [Table/Fig-7].
Correlation between maternal and neonatal zinc.
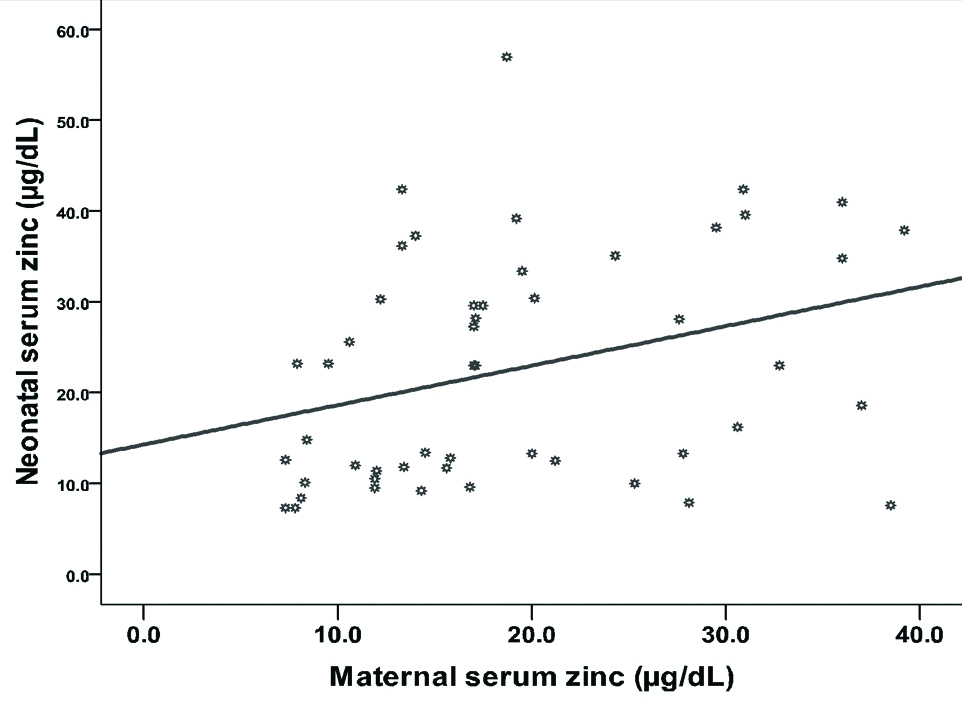
Likewise, significant positive correlation between maternal and neonatal iron has been registered (r=0.312, p=0.026) as shown in [Table/Fig-8].
Correlation between maternal and neonatal iron.
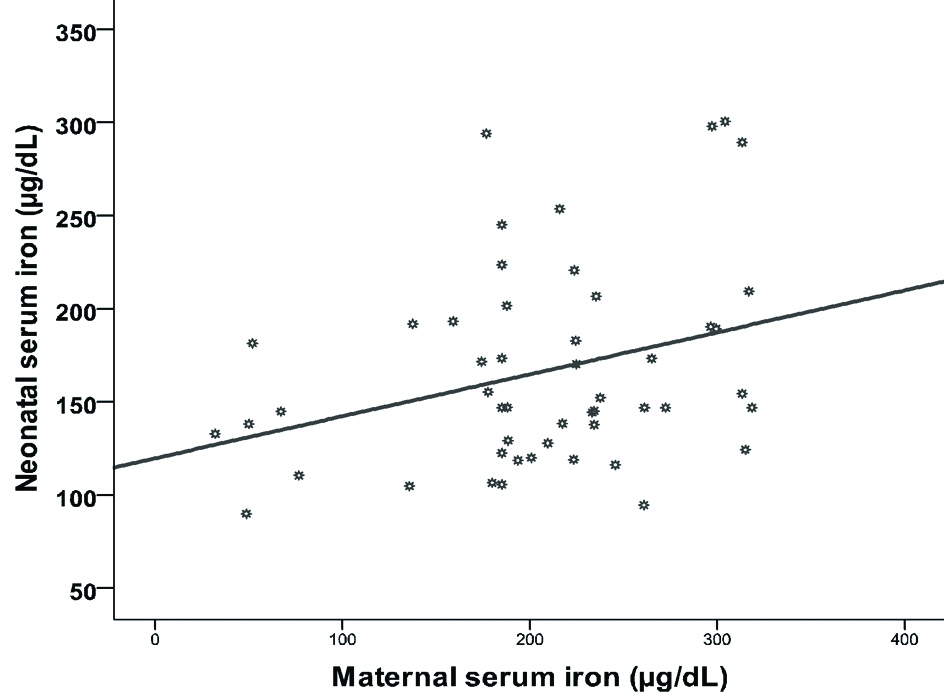
In contrast, significant negative correlation between birth order and neonatal iron (r=-0.511, p<0.001) as shown in [Table/Fig-9].
Correlation between birth order and neonatal iron.
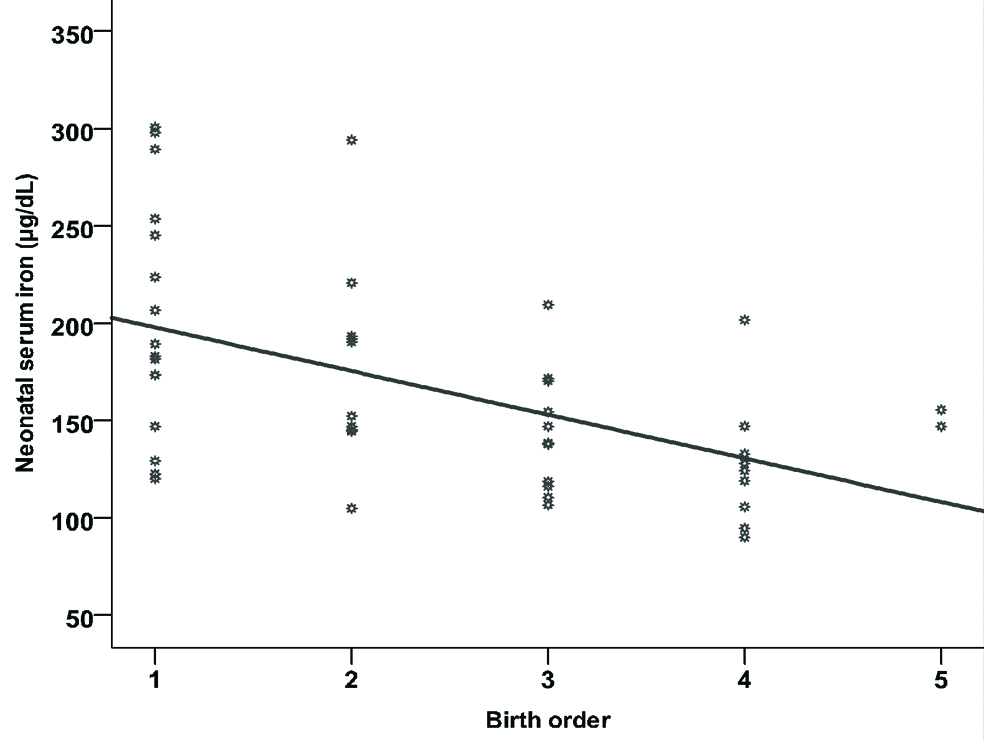
The represented data in [Table/Fig-10] indicated that maternal serum zinc, iron and TIBC are the significant factors that increase neonatal serum zinc, iron and TIBC.
Linear regression models for factors affecting laboratory findings of the studied neonates.
| Neonatal lab | Maternal lab | β | SE | p | 95 CI | R2 |
|---|
| Serum zinc | Serum zinc | 1.043 | 0.088 | <0.001* | 0.867-1.219 | 0.739 |
| Serum iron | Serum iron | 0.741 | 0.042 | <0.001* | 0.658-0.825 | 0.864 |
| Serum TIBC | Serum TIBC | 0.538 | 0.074 | <0.001* | 0.388-0.687 | 0.512 |
Total=51, Pearson correlation, *Significant
Discussion
Trace elements are the corner stone of healthy life [13], their deficiency has long been a major healthcare problem in the Middle East especially, Egypt. During the past three decades, major changes in the economic, political, and social environment have occurred in the region. These changes induced great challenges concerned with diet, nutrition, and health [14].
The main findings of the present study had given a global picture on maternal mineral levels, with low serum zinc levels in almost all the mothers. On the contrary, the levels of iron were high in about 70.6% mothers and TIBC was high in about 45.1%. The lowering serum zinc levels in mothers in the present study is in line with the findings of Khoushabi F et al., [10]. The significant negative correlation between birth order and neonatal iron is considered as a logical finding in the present study i.e., with increasing order of birth there is a decrease in their neonatal iron. This result was explained by Goswmai S et al., who categorized iron as a Type 1 nutrient [15].
It has been demonstrated that iron deficiency increases with the increased order of birth [16]. Birth control measures should be one of the priorities in the programs implemented for maternal and child health. In the current study, significant positive correlation between maternal and neonatal iron was recorded. Maternal iron is the only source of foetal iron, therefore maternal iron status will affect the iron status of the neonate as El Farrash RA et al., concluded [17]. Findings registered a linear relationship of cord blood hemoglobin, iron and ferritin levels with maternal hemoglobin, iron, and ferritin levels supporting that foetus iron is in direct proportion to the levels available in mother. All of these findings were contrary to the findings of Paiva Ade A et al., study, which concluded that the iron nutritional status of pregnant women with iron deficiency or mild anemia does not seem to have a significant effect on the iron levels of their neonates [8].
The study demonstrated significant positive correlation between maternal and neonatal zinc as many studies have reported that with maternal zinc deficiency, there is accompanied neonatal deficiency. Krachler M et al., and Jyotsna S et al., stated that this deficiency may be due to insufficient zinc supplementation during pregnancy [18,19]. However, there is another theory which states that overenthusiastic, uncontrolled supplementation of minerals especially iron during pregnancy could unfavourably affect other minerals because of competitive interaction among trace elements. During the last decade, there has been a tendency to add minerals along with vitamin preparations, which may further exacerbate imbalances [20].
In the present study, it was proved that maternal serum zinc, iron and TIBC are significant factors that increase neonatal serum zinc, iron and TIBC respectively. In other words, neonatal status is a mirror image to maternal one as Martin-Prevel Y et al., mentioned in their study [21]. This points to the need for pre-conceptional nutrition interventions for women of reproductive age, to prevent women from entering pregnancy with low nutritional status. Authors will continue the study on a large group of mothers and their neonates to detect maternal clinical predictors for these results.
Limitation
The limitation of the present study was small sample size due to the absence of suitable fund.
Conclusion
This study provides important insight into the impact of maternal zinc, iron and TIBC concentration on their neonatal levels. Therefore there is a specific need for proper, adequate and balanced micronutrients during pregnancy in order to have healthy neonates.
Total=51
Total=51
Total=51
Total=51
^Independent t-test, #Fisher-Exact test, *Significant
^Independent t-test, #Fisher-Exact test, *Significant, OW-Over Weight, NVD-Normal Vaginal Delivery, CS-Cesarean Section
Total=51, Pearson correlation, *Significant