Plasma Neutrophil Elastase and its Endogenous Inhibitors as Differential Inflammatory Markers for Dengue and Pneumonia
Mamatha Kunder1, V Lakshmaiah2, AV Moideen Kutty3
1 Assistant Professor, Department of Biochemistry, Sri Devaraj Urs Medical College, A Constituent College of Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar, Karnataka, India.
2 Professor, Department of Medicine, Sri Devaraj Urs Medical College, A Constituent College of Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar, Karnataka, India.
3 Professor, Department of Biochemistry, Sri Devaraj Urs Medical College, A Constituent College of Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. AV Moideen Kutty, Pxsrofessor, Department of Biochemistry, Sri Devaraj Urs Medical College, Tamaka, Kolar-563101, Karnataka, India.
E-mail: kuttyavm@gmail.com
Introduction
Neutrophils play a crucial role in protecting the host against microbial pathogens but they produce proteolytic enzymes such as Neutrophil Elastase (NE), the uncontrolled activity of which can destroy tissue and lead to organ failure. Thus, measurement of circulating levels of NE and its endogenous inhibitors may possibly contribute to diagnosis and management of diseases.
Aim
This study was aimed to evaluate the changes in levels of NE, alpha1-Antitrypsin (α1-AT), alpha2-Macroglobulin (α2-MG) as well as elastase in complex with α1-AT (NE-α1-AT complex) in patients with dengue and pneumonia to ascertain if they could be of use as differential inflammatory markers and as adjunct diagnostic parameters in these two distinct disease conditions.
Materials and Methods
This was a comparison study including 152 individuals in which 50 were in dengue group, 50 in pneumonia group, and 52 in control group. NE was measured using N-Succinyl-tri-alanine-p-nitroanilide as substrate. α1-AT, α2-MG and NE-α1-AT complex were estimated by Enzyme-Linked Immunosorbent Assay (ELISA). ANOVA, Kruskal-Wallis test and Pearson’s correlation tests were used to analyse the data. The results were expressed as Mean±SD and p-value <0.001 was considered statistically highly significant.
Results
The result analysis indicated that the dengue and pneumonia patients had significantly higher elastase activity with significantly reduced α1-AT levels compared to controls. α2-MG level were significantly decreased in dengue while the levels were significantly increased in pneumonia.
Conclusion
Significantly elevated levels of NE were observed in patients with dengue and pneumonia. Significantly reduced α1-AT in dengue and significantly increased α2-MG in pneumonia are observations of relevance.
Alpha1-antitrypsin, Alpha2-macroglobulin, Neutrophil elastase- a1-Antitrypsin complex
Introduction
Infectious diseases are considered as the major cause of mortality and morbidity worldwide [1]. Pneumonia and dengue are two such infections, which are rapidly escalating all over the world at an alarming rate. Pneumonia is a severe infection of the airways while dengue is one of the most emerging arthropod-borne viral diseases. Annually, approximately 2 million and 50 million people fall ill due to pneumonia and dengue infection, respectively [2,3].
The clinical manifestations such as fever, respiratory symptoms, and chest x-rays are usually recommended for the diagnosis of pneumonia [4]. On the other hand, viral culture, nucleic acid amplification, and serological assays have been used for the diagnosis of dengue [5]. However, at times such disease specific approaches are insufficient as many infectious diseases share common clinical symptoms. Therefore, it might be of use to have some diagnostic adjunct parameters reflective of progression, diagnosis and severity of diseases. Since these disease conditions are characterised by inflammation, measurements of circulating levels of inflammatory markers would provide vital clues for the underlying causes and contribute to diagnosis and management of diseases.
Neutrophils are the first immune cells to be recruited to sites of infection and play a crucial role in protecting the host against microbial pathogens [6]. They produce proteolytic enzymes such as NE (EC number 3.4.21.37) and the uncontrolled activity which can trigger off destructive processes associated with various chronic diseases. The regulation of proteolytic activity of NE under normal physiological conditions is achieved by inhibitory action of potent endogenous antiproteases, such as alpha1-antitrypsin (α1-AT), α2-macroglobulin (α2-MG) and secretory leucoproteinase inhibitor. These inhibitors form stable enzyme-inhibitor complexes with NE, which are devoid of proteolytic activity and thus helps the host to defend the tissue damage [7,8]. During infection, NE activity is enhanced and surpasses the normal regulatory mechanisms resulting in excessive proteolysis of extracellular matrix proteins causing tissue damage and progress of disease [9].
Studies have shown significant alterations in the levels of NE and its endogenous inhibitors in pneumonia and dengue [9,10]. Since a comparative evaluation of neutrophil enzyme, elastase and its endogenous inhibitors has not been done in bacterial and viral infection, the focus of this study was to evaluate the levels of these molecules among patients diagnosed with dengue (viral infection) and pneumonia (bacterial infection) to gain insights into whether or not these molecules are affected among bacterial and viral infection and also for their utility in either diagnosis or prognosis.
Materials and Methods
Study Design
The present study was a comparative study carried out from June 2016 to February 2017 at the Department of Biochemistry of Sri Devaraj Urs Medical College, constituent college of Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India. The study involved randomly selected subjects admitted in the Medicine department of RL Jalappa Hospital and Research Centre, the teaching hospital of the medical college. Every enrolled patient or their relatives gave informed written consent to participate in the study. Ethical approval for the study was obtained from Institutional Ethical Committee (DMC/KLR/MEU/IEC/262/2011-12) and the study complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration.
A total of 152 participants were recruited and divided into three groups- 52 in control group, 50 in dengue group and 50 in pneumonia group.
Patient Selection Criteria
Dengue: A total 50 patients who were positive for IgM and/or IgG were included. Samples were obtained on day 5-7 after the onset of fever. Patients with acute and chronic infections, T2DM, chronic diseases such as cardiac diseases, malignancy, stroke, Chronic Obstructive Pulmonary Disease (COPD), liver disorders, acute renal failures, and history of smoking were excluded from the study.
Pneumonia: A total of 50 clinically and radiologically confirmed cases of both hospital and community acquired pneumonia were included in the study. Bacterial pneumonia was confirmed by culture sensitivity. The patients with other infections, active pulmonary tuberculosis, COPD, malignancy, liver and renal dysfunction, pregnancy, T2DM were excluded from the study.
Sample Collection
Six milliliters of fasting blood was collected under complete aseptic precautions from an antecubital vein into tubes containing sodium fluoride (for FBS), EDTA (for HbA1c, haematologic studies); Sodium Heparin (for NE, α1-AT, α2-MG and NE-α1-AT complex estimation). For investigations like C-Reactive Protein (CRP), renal function and liver function tests blood was collected in tubes without anticoagulant. The blood samples were centrifuged for 15 minutes at 3000 rpm within two hours of collection. After centrifugation, serum and plasma were separated and aliquots were stored at -70°C until assayed. Samples were thawed at room temperature, vortexed and centrifuged before analysis.
Assays: Basic blood chemistry measurements were done by standard methods using Vitros 250 Dry chemistry analyser (Ortho Clinical Diagnostics). Complete Blood Count (CBC) was performed by Beckman-Coulter, an automatic blood cell counter. Serum CRP estimation was done by rapid latex slide tests. Plasma elastase was estimated using Succinyl tri- L-alanyl-p-nitroanilide (STANA, from SIGMA chemicals) as substrate at 410 nm as per the procedure described by Beith J et al., [11]. Plasma α1-AT and α2-MG were analysed using ELISA kit purchased from Immunology Consultants laboratory, Inc, USA. NE-α1-AT complex was quantified by ELISA (Calbiochem).
Statistical Analysis
The data was statistically analysed by SPSS software version 22 (licensed version). The results are expressed as Mean±SD. Differences between groups were analysed using ANOVA (Analysis of Variance) with post-hoc test for normally distributed parameters and Kruskal-Wallis test for not- normally distributed parameters. Pearson’s correlation coefficient was used to analyse the correlation between continuous variables. A p-value ≤0.05 was considered statistically significant and <0.001 as highly significant.
Results
Baseline characteristics of the study groups are presented in the [Table/Fig-1]. The mean age was 48 years in control and dengue group while it was 57 years in pneumonia group. Gender distribution among the studied groups was statistically non-significant. There were significant changes in haematological parameters between control and patient groups which reflect the clinical symptoms.
Baseline characteristics of study and control groups.
| Variables | Control (n=52) | Dengue (n=50) | Pneumonia (n=50) |
|---|
| Age (years) | 48.48±6.39 | 48.50±4.34 | 57.5±16 |
| Gender (male/female) | 32/20 (61.5%/38.5%) | 30/20 (60%/40%) | 22/28 (44%/56%) |
| HCT (%) | 42.27±3.63 | 43.01±5.22 | 35.13±5.35 |
| PL count (103/L) | 272.23±61.24 | 71.94±39.17 | 267.64±40.04 |
| Total WBCs (103/L) | 7.04±1.02 | 4.38±1.81 | 14.25±2.75 |
| Neutrophils (%) | 51.27±7.37 | 40.68±12.37 | 82.42±6.86 |
| Lymphocytes (%) | 37.86±6.62 | 33.99±9.20 | 7.21±2.68 |
| Monocytes (%) | 5.01±1 | 14.24±6.09 | 6.64±1.73 |
| CRP (μg/mL) | 0 | 22.92±7.6 | 28.68±5.54 |
Values are expressed as Mean±SD
Plasma levels of elastase activity, α1-AT, α2-MG and NE-α1-AT complex in the study groups are depicted in the [Table/Fig-2]. The mean plasma elastase activity were significantly higher in both dengue (0.78±0.20) and pneumonia (0.70±0.09) patients than in healthy subjects (0.35±0.21) with p-value <0.001. On the other hand, the mean α1-AT levels were decreased significantly in patients with dengue (66.25±7.75) compared to controls (123.35±25.66) and in patients with pneumonia (116.06±63.91). However, the comparison of levels of α1-AT between controls and pneumonia did not reveal any significance. The mean α2-MG levels were significantly decreased in patients with dengue (162.75±24.16) and increased in patients with pneumonia (262.73±46.99) when compared to controls (209.42±31.15). When plasma NE-α1-AT complex was compared, the pneumonia cases exhibited significant increase reflective of the increased levels of NE and α1-AT, while the dengue cases presented marginal increase which was not statistically significant (p=0.068).
Plasma elastase activity, α1-AT, α2-MG and NE-α1-AT complex in the study groups.
| Disease | Plasma Elastase Activity (U/mL) | Plasma α1-AT (mg/dL) | Plasma α2-MG (mg/dL) | Plasma NE-α1-AT complex (ng/mL) |
|---|
| Control | 0.35±0.21 | 123.35±25.66 | 209.42±31.15 | 214.89±13.05 |
| Dengue | 0.78±0.20 | 66.25±7.75 | 162.75±24.16 | *238.38±62.47 |
| Pneumonia | 0.70±.09 | *116.06±63.91 | 262.73±46.99 | 356.30±31.81 |
Values are expressed as Mean±SD. *Statistically non-significant
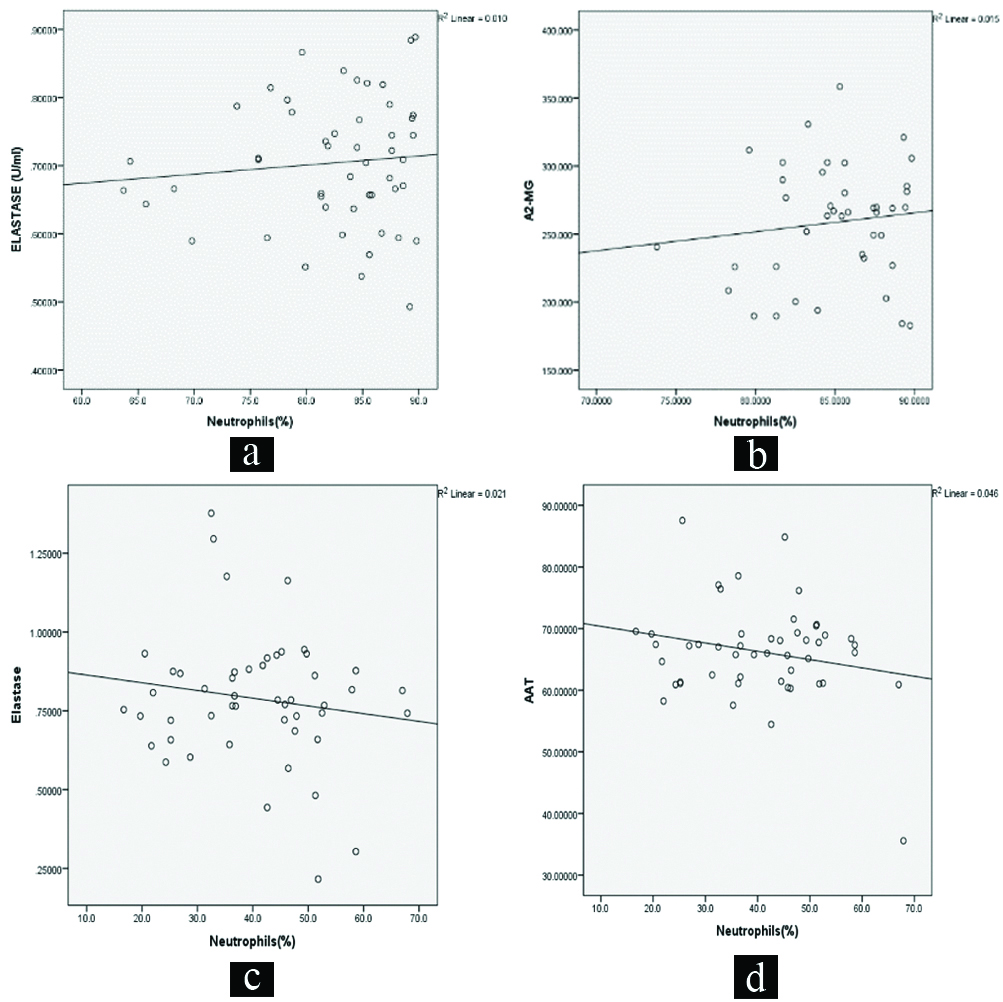
Correlation studies were carried out with parameters which were significantly increased or decreased in comparison to the normal values. In pneumonia, the base parameter neutrophil counts correlation with NE and α2-MG presented positive associations. While neutrophil counts with elastase and α1-AT correlated negatively in dengue patients [Table/Fig-3].
Linear correlation plots: a) between neutrophil count and NE in pneumonia (r=0.098, p=0.500); b) between neutrophil count and α2-MG in pneumonia (r=0.121, p=0.449); c) between neutrophil count and NE in dengue (r=-0.146, p=0.315); d) between neutrophil count and α1-AT in dengue (r=-0.215, p=0.139).
Discussion
Pathophysiologic differences are seen in the degree of tissue destruction between dengue and pneumonia infection. Bacterial pneumonia is reflected by neutrophilia whereas dengue is characterised by neutropenia. Pro-inflammatory cytokines produced in response to bacterial and dengue virus infection are shown to activate neutrophil functions and thus are involved in the migration and activation of neutrophils [9,10,12]. The most significant observation of this study was elevated levels of elastase activity in dengue and pneumonia patients than in normal subjects and this could be attributed to enhanced neutrophil activation and degranulation. This finding also validates the central role of NE in acute pathogenesis and support future exploration of the therapeutic window. This finding is in accordance with the previous studies conducted [9,10].
Though an increased level of α1-AT was expected in the patient groups to counter the elevated elastase activity as natural defence mechanism, the level of the inhibitor was decreased in disease groups which were highly significant in dengue and statistically not significant in pneumonia cases. It is known that the patients suffering from infection exhibit an increase in oxidative stress and generate free radicals [13]. These free radicals have been shown to destroy α1-AT [10]. Accordingly the decrease in the level of this inhibitor observed in this study may be due to the destruction of this molecule by free radicals. However, a possible explanation for the drastic decrease in dengue infection could be attributed to neutropenia caused by increased destruction of neutrophils and release of excessive Reactive Oxygen Species (ROS) which in turn destroy α1-AT [14,15]. Though in the current study an analysis of ROS had not been done, the data available in the literature is reflective of explanation cited above and notwithstanding the possibilities of involvement of gene expression of α1-AT.
With regard to the levels of α2-MG, the patients suffering from pneumonia had higher levels in comparison to controls. α2-MG is shown to possess antiplasmin activity and abnormalities in coagulation cascade observed in pneumonia could be attributed to this [16]. In contrast the levels of α2-MG were decreased in dengue almost reflecting the pattern of α1-AT. The levels of both α1-AT and α2-MG are decreased significantly in dengue while the pattern of change was reflected only in α2-MG in case of pneumonia. The complex formation between NE and α1-AT was increased in pneumonia which was reflective of the increased NE and near normal levels of α1-AT. There was no significant increase in the levels of NE-α1-AT complex in dengue patients as expected on account of the decreased levels of α1-AT. Thus, it is evident that the decreased α1-AT observed in dengue fever is distinct and reflective. This study opens up a concept that in bacterial infection there is an increase in α2-MG and in viral infection decrease in α1-AT. Though, it is premature to emphasis on this concept, the question whether these are characteristics of host response in bacterial and viral diseases or otherwise need to be studied.
In this study, a significant elevation in elastase and α2-MG were observed in pneumonia and a significant increase in the levels of elastase along with a decrease in its primary inhibitor α1-AT in dengue patients which were correlated with neutrophil count. The outcomes of the correlation study indicate that the measurements of α2-MG in pneumonia and α1-AT levels in dengue are relevant as adjunct diagnostic parameters along with the routine clinical examinations and laboratory investigations. Further, in dengue group a negative correlation between neutrophils and elastase was observed in this study which signifies the protective role of neutrophils against dengue virus and also destructive effect of elastase leading to further complications seen in severe forms of dengue.
Conclusion
This study establishes an elevated NE activity in both viral and bacterial infections in conformity with characteristics of inflammatory processes. A significant decrease observed in the levels of α1-AT in dengue, a viral infection and an increase in the α2-MG levels in bacterial pneumonia are of significance as differential inflammatory markers in these disease conditions. However, further studies have to be conducted on other viral and bacterial diseases to confirm whether these findings are unique to these infectious agents or otherwise.
Values are expressed as Mean±SD
Values are expressed as Mean±SD. *Statistically non-significant
[1]. Markanday A, Acute phase reactants in infections: evidence-based review and a guide for cliniciansOpen forum infectious diseases 2015 Oxford University Press10.1093/ofid/ofv09826258155 [Google Scholar] [CrossRef] [PubMed]
[2]. Huang H, Ideh RC, Gitau E, Thezenas ML, Jallow M, Ebruke B, Discovery and validation of biomarkers to guide clinical management of pneumonia in African childrenClin Infect Dis 2014 58(12):1707-15.10.1093/cid/ciu20224696240 [Google Scholar] [CrossRef] [PubMed]
[3]. Conroy AL, Gelvez M, Hawkes M, Rajwans N, Tran V, Liles WC, Host biomarkers are associated with progression to dengue haemorrhagic fever: a nested case-control studyInt J Infect Dis 2015 40:45-53.10.1016/j.ijid.2015.07.02726255888 [Google Scholar] [CrossRef] [PubMed]
[4]. Evertsen J, Baumgardner DJ, Regnery A, Banerjee I, Diagnosis and management of pneumonia and bronchitis in outpatient primary care practicesPrim Care Respir J 2010 19(3):23710.4104/pcrj.2010.0002420490437 [Google Scholar] [CrossRef] [PubMed]
[5]. Parkash O, Shueb RH, Diagnosis of dengue infection using conventional and biosensor based techniquesViruses 2015 7(10):5410-27.10.3390/v710287726492265 [Google Scholar] [CrossRef] [PubMed]
[6]. Kolaczkowska E, Kubes P, Neutrophil recruitment and function in health and inflammationNature Reviews Immunology 2013 13:159-75.10.1038/nri339923435331 [Google Scholar] [CrossRef] [PubMed]
[7]. Keiko F, Yasuhiko S, Noriaki I, Ritsuko Y, Masanori O, Masakuni O, Release of neutrophil elastase and its role in tissue injury in acute inflammation: effect of the elastase inhibitor, FR134043European Journal of Pharmacology 1999 374(1):117-25.10.1016/S0014-2999(99)00268-X [Google Scholar] [CrossRef]
[8]. Yamanouchi H, Fujita J, Hojo S, Yoshinouchi T, Kamei T, Yamadori I, Neutrophil elastase – alpha-1- proteinase inhibitor complex in serum and BAL fluid in patients with pulmonary fibrosisEur Respir J 1998 11:120-25.10.1183/09031936.98.110101209543280 [Google Scholar] [CrossRef] [PubMed]
[9]. Hiroto M, Katsunori Y, Hiroshi M, Kenji T, Masamitsu N, Shigeru K, Association of plasma neutrophil elastase levels with other inflammatory mediators and clinical features in adult patients with moderate and severe pneumoniaRespiratory Medicine 2007 101:1521-28.10.1016/j.rmed.2007.01.00117296292 [Google Scholar] [CrossRef] [PubMed]
[10]. Juffrie M, Van Der Meer GM, Hack CE, Haasnoot K, Sutaryo Veerman AJP, Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulationInfection and Immunity 2000 68:702-07.10.1128/IAI.68.2.702-707.200010639436 [Google Scholar] [CrossRef] [PubMed]
[11]. Bieth J, Spiess B, Wermuth CG, The synthesis and analytical use of a highly sensitive and convenient substrate of elastaseBiochemical Medicine 1974 11:350-57.10.1016/0006-2944(74)90134-3 [Google Scholar] [CrossRef]
[12]. Halstead SB, Antibody, macrophages, dengue virus infection, shock and hemorrhage: a pathogenic cascadeRev Infect Dis 1989 11:830-39.10.1093/clinids/11.Supplement_4.S8302665015 [Google Scholar] [CrossRef] [PubMed]
[13]. Pohanka M, Role of oxidative stress in infectious diseases- A reviewFolia Microbiol 2013 58(6):503-13.10.1007/s12223-013-0239-523504625 [Google Scholar] [CrossRef] [PubMed]
[14]. Soundravally R, Sankar P, Bobby Z, Hoti SL, Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidationPlatelets 2008 19:447-54.10.1080/0953710080215528418925513 [Google Scholar] [CrossRef] [PubMed]
[15]. Siddiqui T, Zia MK, Ali SS, Rehman AA, Ahsan H, Khan FH, Reactive oxygen species and antiproteinasesArchives of Physiology and Biochemistry 2016 122(1):1-7.10.3109/13813455.2015.111552526699123 [Google Scholar] [CrossRef] [PubMed]
[16]. Eric MB, Michael CR, MinJae L, Stephanie LS, Derek CA, Lan K, Prevalence and significance of Coagulation abnormalities in Community- Acquired PneumoniaMol Med 2009 15(11-12):438-45.10.2119/molmed.2009.0009119753144 [Google Scholar] [CrossRef] [PubMed]