Sclerosing Extramedullary Haematopoietic Tumour Presenting as Bilateral Gluteal Abscess in a Patient with Chronic Myelogenous Leukaemia
KS Sunil Kumar1, N Geetha2, L Swetha3, Prabu4
1 Consultant, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
2 Senior Consultant, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
3 Senior Registrar, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
4 Consultant, Department of Hematology, Apollo Hospitals, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: KS Sunil Kumar, Consultant, Department of Histopathology, Apollo Hospitals, Greams Road, Chennai-600006, Tamil Nadu, India.
E-mail: pathsunil@gmail.com
Sclerosing Extramedullary Haematopoietic Tumours (SEMHT) are found to be associated with Chronic Myeloproliferative Neoplasms most commonly reported in the skin, lacrimal system, orbit, omentum and liver. A 26-year-old male with a known history of Chronic Myelogenous Leukaemia (CML) sought medical attention for non-healing ulcerated lesions on both gluteal regions. Histopathological examination revealed patchy skin ulceration with dermal abscess formation, dense-periadnexal and perivascular extramedullary haematopoietic elements comprising of erythroid progenitors, granulocytes with all stages of maturation and atypical megakaryocytes extending into subcutaneous fat. Immunohistochemistry confirmed the lineage of erythroid progenitors by Glycophorin, granulocyte lineage by Myeloperoxidase and megakaryocytes by CD61 staining. CD34, CD30 and CD15 immunoreactivity were negative. Keeping this differential diagnosis in mind is essential while reporting a sclerotic lesion with extramedullary haematopoietic elements in a clinical setting of Chronic Myeloproliferative Neoplasms to prevent inaccurate diagnosis and unnecessary surgeries.
Atypical megakaryocytes, Chronic myeloproliferative neoplasms, Lacrimal system
Case Report
A 26-year-old male was diagnosed with CML in December 2012 with bone marrow tests followed by confirmation with FISH analysis of 200 interphase cells displaying BCR/ABL1 fusion positive in 100% of cells. He was started on "Imatinib" and was compliant with treatment for first two years. After then he was on irregular treatment and was lost to follow-up. He developed a small blister in the left gluteal region in the month of February 2017 which gradually increased in size in spite of regular dressing.
Clinical examination revealed a 10×8 cm ulcerated lesion in the left gluteal region with blackish discolouration, irregular margins, purulent and foul-smelling discharge. The right gluteal region also showed a non-ulcerated, tender, indurated ovoid nodule measuring 3×2 cm which was not fixed to the underlying structures.
On admission, his laboratory values included a low Haemoglobin count of 9.1 g/dL, low Packed Cell Volume of 33%, total white cell count of 8.55×109/L, Platelet count of 731×109/L, elevated Erythrocyte Sedimentation Rate (automated-Westergren method) 32 mm/1st hour. Differential Leukocyte Count revealed absolute basophilia and eosinophilia; normocytic hypochromic RBCs and increased platelets with giant forms suggestive of myeloproliferative neoplasms.
He was also advised for bone marrow aspiration cytology and trephine biopsy to assess disease activity and fibrosis. However, the patient refused to undergo bone marrow evaluation citing personal reasons. Clinical differential diagnosis at this point of time was cutaneous myeloid leukaemic infiltrates in the clinical setting of CML.
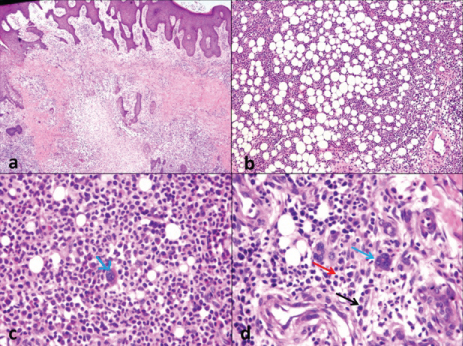
Under endotracheal general anaesthesia, lesion was excised from bilateral gluteal regions. The excision biopsy from the left gluteal region revealed a 10×8×2.5 cm area of infected ulcerated lesion with irregular margins, areas of necrosis, surrounding areas of induration, not fixed to underlying structures with tenderness; and the right gluteal region biopsy revealed a 3×2×1.5 cm indurated lesion with tenderness, regular margins, non ulcerated, oval in shape, not attached to underlying structures. Histopathological examination revealed patchy skin ulceration with bilateral gluteal dermal abscess formation, dense periadnexal and perivascular extramedullary haematopoietic elements [Table/Fig-1a], comprising of erythroid progenitors [Table/Fig-1b], granulocytes in various stages of maturation [Table/Fig-1b] and atypical megakaryocytes [Table/Fig-1c,d]. Amidst the extramedullary haematopoietic elements, the dermis shows proliferating fibroblasts with extensively sclerotic stroma extending into the subcutaneous fat.
a) Subcutaneous nodular extramedullary hematopoietic elements-H&E, 100x; b) Erythroid and Myeloid precursors in varying stages of maturation-H&E, 100x; c) Atypical megakaryocytes (blue arrow)-H&E, 400x; d) Atypical megakaryocytes (blue arrow) amidst the erythroid (red arrow) and myeloid precursors (black arrow)-H&E, 400x.
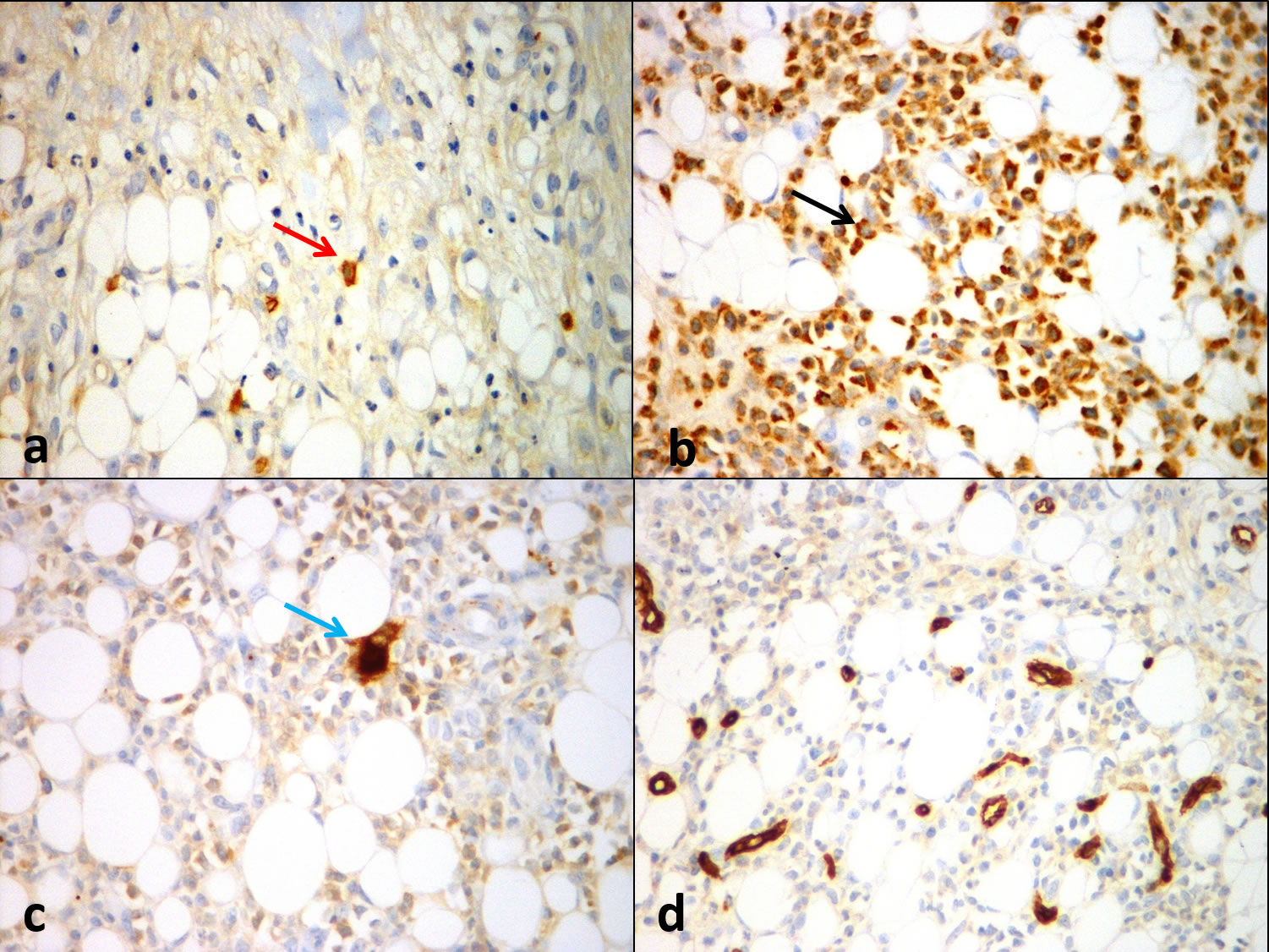
Immunohistochemistry confirmed the lineage of haematopoietic elements. Erythrocyte precursors stained positively with Glycophorin [Table/Fig-2a], Granulocyte precursors stained positively with Myeloperoxidase (MPO) [Table/Fig-2b] and Megakaryocytes stained positively with CD61 [Table/Fig-2c]. The atypical cells were negative for CD34 [Table/Fig-2d], CD30 and CD15 thereby excluding the possibilities of Acute Leukaemia and Hodgkin lymphoma respectively. Based on the morphological findings and immunohistochemical findings, the diagnosis of Sclerosing Extra Medullary Haematopoietic Tumour was made. The microbial cultures work-up after the debridement of wounds revealed Methicillin-Sensitive Staphylococcus Aureus (MSSA) and Extended Spectrum Beta Lactamase (ESBL) positivity. He was started on Inj. Invanz 1 g/20 mL OD for seven days and T.sporidex 500 mg OD for seven days. The patient has remained well since discharge. He continued with the Imatinib (Levin 400 mg once daily) until July 2017 when he reviewed with us. The patient was not willing to undergo BCR/ABL gene status in the gluteal lesion and hence it was not attempted. Bone marrow performed during this time revealed accelerated phase of CML for which he was switched to Nilotinib 200 mg bd up until now. After the introduction of Nilotinib, he has recovered from gluteal ulcer and was keeping well for the past one year.
a) Positive immuhistochemical staining with Glycophorin demonstrating erythroid precursors (red arrow), 400x; b) MPO immunostain demonstrating myeloid precursors (black arrow), 400x; c) CD61 immunostain demonstrating atypical megakaryocytes (blue arrow), 400x; d) Negative staining with CD 34, 400x.
Discussion
SEMHT is a rare neoplasm associated with chronic myeloproliferative disorders or Myelofibrosis [1]. Author describe an unusual case of SEMHT presenting as bilateral gluteal abscess in a setting of CML. Awareness of this entity and differentiating it from other pathological conditions like lymphoma, sarcoma and carcinoma is pertinent to avoid unnecessary interventions. Histologically, SEMHT is characterised by prominent sclerotic background with thick collagen fibres, fibroblastic proliferation, variable trilineage extramedullary haematopoietic elements including atypical megakaryocytes [2].
This tumour is unique and shows distinct histopathological features like sclerosis which is prominent with deposition of thick collagen, various elements of trilineage haematopoiesis as well as atypical megakaryocytes and varying proportions of myeloid and erythroid precursors [3-6]. It may be solitary or multiple seen predominantly on serosal and retroperitoneal regions. It has also been reported in skin, lacrimal system, orbit, omentum and liver. The present patient presented with bilateral gluteal abscess in a clinical setting of BCR/ABL1 positive CML on irregular treatment. Histopathological examination is considered to be the gold standard in the diagnosis of SEMHT [3,5-7]. The typical histopathological features of SEMHT like dense fibrotic background and trilineage extramedullary haematopoietic elements, eosinophilic cytoplasm with “ink blot-like” nuclei of dysplastic or atypical megakaryocytes is considered classical for diagnosis [6]. Localisation of haematopoiesis in tissues other than bone marrow and association with chronic idiopathic myelofibrosis is described as EMH which has similar clinical manifestations as SEMHT. The classic extramedullary haematopoiesis is distinguished from SEMHT by absence of prominent sclerotic background and very cellular marrow like appearance of extramedullary haematopoietic elements. Pathology of sclerosis in tumour is due to secretion of cytokines by the clonal megakaryocytes which in turn induces fibroblasts. Other conditions to be considered in the differential diagnosis include Hodgkin lymphoma, Histiocytic lymphoma, Granulocytic sarcoma, multinucleate giant cells of Granulomatous inflammatory lesions, Myelolipoma, Sclerosing liposarcoma, Myxofibrosarcoma, Sarcomatoid carcinoma and Pleomorphic sarcoma which can have similar kind of sclerosis, fibroblasts, atypical giant cells and entrapped fat [3,4]. Karaarslan S et al., described a case of SEHMT mimicking Intra-abdominal Sarcoma in which diagnosis was made based on immunohistochemistry similar to the present case [8]. Immunohistochemical analysis with CD15 and CD30 are helpful in distinguishing atypical megakaryocytes from Reed-Sternberg cells and absence of haematopoietic elements with presence of Pancytokeratin immunostain positivity helps in identifying a carcinoma. Myeloid sarcoma, a tumour of myeloid blastic cells is an important differential diagnosis for atypical megakaryocytes of SEMHT in this clinical setting of myeloproliferative disorder. Immunohistochemistry for CD34 and CD117 would assist us in this diagnostic pitfall which will be positive in the blasts. Sclerosing liposarcomas are positive for MDM2 and CDK4 while Low-Grade Fibromyxoid Sarcomas (LGFMS) are positive for MUC4. Authors were able to narrow down the diagnosis after a thorough clinicopathological correlation and immunohistochemical analysis.
This patient presented with abscess in the gluteal region. Clinical history disclosed CML background. The diagnosis was confirmed with IHC technique using Glycophorin, MPO, CD61, CD34, CD30 and CD15 [9-11]. Classic light microscopy together with few IHC markers helped us to narrow down the diagnosis. So, the other markers have not been performed.
Conclusion
In conclusion, the objective of this case presentation is to highlight the importance of this less known benign entity in the realm of myeloproliferative disorders. Sclerosing Extramedullary Haematopoietic Tumours (SEMHT) can mimic clinically, radiologically and histologically various pathological conditions as enumerated above and hence this condition should be borne in mind while making a diagnosis, especially in a patient with history of chronic myeloproliferative disorder. Awareness of the significance of sclerosis, fibroblastic proliferation and atypical megakaryocytes in a setting of EMH should heighten the suspicion for SEMHT to minimize errors and unnecessary surgical intervention.
[1]. Shinde SV, Shenoy AS, Balsarkar DJ, Shah VB, Omental Sclerosing extramedullary hematopoietic tumours in Janus kinase-2 negative myelofibrosis: Caveat at frozen sectionIndian J Pathol Microbiol 2014 57:480-82.10.4103/0377-4929.13878525118752 [Google Scholar] [CrossRef] [PubMed]
[2]. Gu MJ, Sclerosing extramedullary hematopoietic tumour presenting as an inguinal mass in a patient with primary myelofibrosis: a diagnostic pitfallInt J of Clin and Exp Pathol 2015 8:3381-83. [Google Scholar]
[3]. Remstein ED, Kurtin PJ, Nascimento AG, Sclerosing extramedullary hematopoietic tumour in chronic myeloproliferative disordersAm J Surg Pathol 2000 24:51-55.10.1097/00000478-200001000-0000610632487 [Google Scholar] [CrossRef] [PubMed]
[4]. Gualco G, Ojopi EB, Chioato L, Cordeiro DL, Negretti F, Bacchi CE, Postsplenectomy Sclerosing extramedullary hematopoietic tumour with unexpected good clinical evolution: morphologic, immunohistochemical, and molecular analysis of one case and review of the literatureAppl Immunohistochem Mol Morphol 2010 18:291-95.10.1097/PAI.0b013e3181c69bfb20042850 [Google Scholar] [CrossRef] [PubMed]
[5]. Beckman EN, Oehrle JS, Fibrous hematopoietic tumours arising in agnogenic myeloid metaplasiaHum Pathol 1982 13:804-10.10.1016/S0046-8177(82)80076-2 [Google Scholar] [CrossRef]
[6]. Kwon Y, Yu E, Huh J, Lee SK, Ro JY, Sclerosing extramedullary hematopoietic tumour involving lesser omentum and ligamentum teres in liver explantAnn Diagn Pathol 2004 8:227-32.10.1053/j.anndiagpath.2004.04.00615290675 [Google Scholar] [CrossRef] [PubMed]
[7]. Yang X, Bhuiya T, Esposito M, Sclerosing extramedullary hematopoietic tumourAnn Diagn Pathol 2002 6:183-87.10.1053/adpa.2002.34574 [Google Scholar] [CrossRef]
[8]. Karaarslan S, Nese N, Oncel G, Ozsan N, Akalin T, Kaplan H, Sclerosing extramedullary hematopoietic tumour mimicking intra-abdominal sarcomaJ of Pathol Transl Med 2015 49:335-38.10.4132/jptm.2015.04.2226072949 [Google Scholar] [CrossRef] [PubMed]
[9]. Li R, Reddy VV, Palmer CA, Extramedullary hematopoiesis: an unusual finding in subdural hematomasCase Rep Pathol 2011 2011:718585Published online 2011 Sep 2710.1155/2011/71858522937391 [Google Scholar] [CrossRef] [PubMed]
[10]. Amanzada A, Malik IA, Nischwitz M, Sultan S, Naz N, Ramadori G, Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflammed liverHistochemistry and Cell Biology 2011 135:305-15.10.1007/s00418-011-0787-121327394 [Google Scholar] [CrossRef] [PubMed]
[11]. Chuang S, Yung Y, Li C, von Willebrand is the most reliable immunohistochemical marker for megakaryocytes of myelodysplastic syndrome and chronic myeloproliferative disordersAm J Clin Pathol 2000 113:506-11.10.1309/9Q6D-GXHU-N1K9-T6BH10761451 [Google Scholar] [CrossRef] [PubMed]