Type 2 Diabetes Mellitus is growing epidemic globally. In India, 69.2 million individuals had diagnosed diabetes (2015) which is expected to increase to 123.5 million by the year 2040 [1]. T2DM is known to increase the risk of bone fracture in adult males and females especially above 50 years of age [2]. We previously reported that T2DM is associated with increased osteoporosis at hip and spine in otherwise healthy T2DM patients from India [3,4]. However, other reports suggested no differences in Bone Mineral Density (BMD) in Asian Indian with T2DM compared to healthy volunteers [5]. World Health Organisation (WHO) recommends T-score of less than -2.5 SD at femoral neck for diagnosis of osteoporosis [6].
T2DM is associated with increased cortical porosity and alteration in bone remodelling [7]. Increased cortical porosity reported in long bones like distal radius and distal tibia is associated with a fragility fracture in diabetic post-menopausal women [8]. The Rotterdam study in T2DM patients reported higher incidence of wrist fracture in patients with inadequately controlled diabetes (0.0078 per 2134 person-years) than adequately controlled (0.0042 per 2165 person-years) or no diabetes (0.0052 per 46130 person-years) [9].
Though increased risk of fractures involves both axial and appendicular bones, there is relative lack of studies from India assessing risk of bone density of appendicular long bone in T2DM patients. We aimed to understand the prevalence of distal radius osteoporosis, correlate it to the spine and hip BMD and identify the factors determining the bone loss in otherwise healthy adults with T2DM.
Materials and Methods
The cross-sectional, observational study was conducted in patients with T2DM, at a tertiary care centre in Rajasthan. The study was conducted with ethical principles of Declaration of Helsinki. An informed consent was obtained from all the participants. The methodology applied in this study is similar to our previously published report [3] and is also briefed here. This study is the report on the analysis of distal radius BMD in relation to BMD at spine and/or hip as well as its correlation to various factors like age, gender and vitamin D levels.
Between January 2015 to December 2016, consecutive 200 patients with T2DM were enrolled. All the patients were recruited during a defined period. Included patients were aged above 50 years, either gender with T2DM. Patients were diagnosed as T2DM when HbA1c levels were ≥6.5% or fasting plasma glucose levels were ≥126 mg/dl. Patients above 50 years of age were included as T2DM is more common in middle age adults and bone loss escalates after middle-ages increasing the risk of fractures. Postmenopausal women also are at increased risk of osteoporosis which is attended by 50 years in most of the women. Also, previous studies have included adults above 50 years [3]. We excluded patients with any diabetes-related complications, chronic smokers, chronic medical disorders like chronic kidney disease, thyroid disorders, chronic pancreatitis, etc., primary hyperparathyroidism, known bone and mineral disorders, malignancy, malabsorption syndrome, patients treated with corticosteroid, immunosuppressants, anticonvulsants, thiazolidinediones, or supplements of calcium and vitamin D.
Enrolled patients were subjected to clinical, biochemistry and BMD evaluations. We recorded data like age, gender, height, weight. Body-mass index (BMI, kg/m2) was calculated by the standard formula. Biochemistry evaluation included fasting and post-prandial blood sugar, serum calcium, phosphorus, total proteins, Alkaline Phosphatase (ALP). Hormonal assays performed were intact Parathyroid Hormone (iPTH), thyroid hormones, serum vitamin D and testosterone.
BMD was assessed at spine; hip and distal radius using DXA scans (Hologic explorer DXA system, USA). With proper positioning at each site, a trained technician performed calibration before taking the scans of each patient. T-scores were calculated. T-score of ≤2.5 and −1.0 and −2.49 was considered as osteoporosis and osteopenia respectively. We defined overall osteoporosis prevalence by taking consideration of T-score of ≤2.5 at spine and/or hip.
Statistical Analysis
Data were presented as mean and standard deviation for continuous and as frequency and percentages for categorical variables. Student’s t-test and Chi-square tests were used to detect statistically significant differences in comparisons. One-way analysis of variance (ANOVA) was used to compare continuous data in more than two groups. Pearson coefficient was determined to study the correlations. The p-value <0.05 was considered statistically significant.
Results
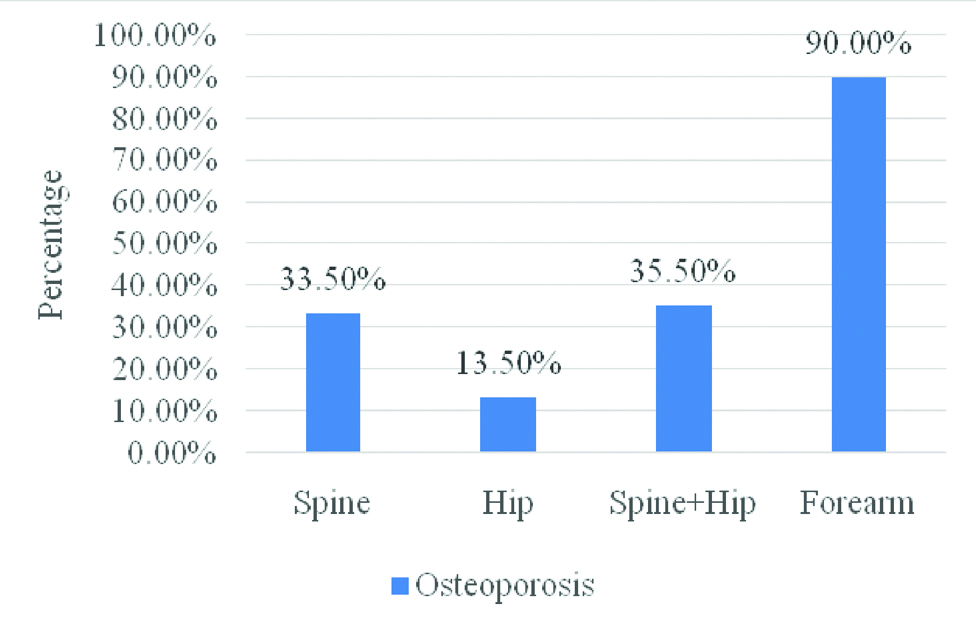
In 200 patients with T2DM, overall (spine and hip) prevalence of osteoporosis (T-score of ≤-2.5) was 35.5% whereas it was 90.0% at distal radius [Table/Fig-1]. Patients were divided in two groups as those with osteoporosis at distal radius (n=180, 90.0%) and without osteoporosis (n=20, 10.0%). Baseline characteristics in two groups are shown in [Table/Fig-2]. Mean age (p=0.768) and group-wise age distribution (p=0.510) was not significantly different in two groups. Presence of distal radius osteoporosis was observed in an equal number of males and females (50.0% each, p=0.203). BMI was significantly higher in patients without osteoporosis at distal radius than those with osteoporosis (28.0±5.3 vs 25.7±4.7 respectively, p=0.048). In biochemical and hormonal assays, except for ALP levels being significantly greater in patients without osteoporosis (p=0.043), there was no statistical difference for any of the parameter as shown in [Table/Fig-2].
Osteoporosis prevalence defined by T-score at different sites.
Baseline characteristics of patients with or without osteoporosis at distal radius.
| Characteristics | Osteoporosis (n=180) | No Osteoporosis (n=20) | p-value |
|---|
| Age (years) | | | |
| Mean±SD | 63.8±6.0 | 63.4±6.7 | 0.768 |
| Age groups (years) | | | |
| 51 to 60 | 43 (23.9) | 7 (35.0) | 0.510 |
| 61 to 70 | 112 (62.2) | 10 (50.0) |
| >70 | 25 (13.9) | 3 (15.0) |
| Gender | | | |
| Male | 90 (50.0) | 13 (65.0) | 0.203 |
| Female | 90 (50.0) | 7 (35.0) |
| BMI (kg/m2) | 25.7±4.7 | 28.0±5.3 | 0.048 |
| Biochemistry | | | |
| Calcium (mg/dL) | 9.0±0.6 | 8.9±0.5 | 0.586 |
| Phosphorus | 3.6±0.6 | 3.4±0.5 | 0.063 |
| ALP (U/L) | 80.2±28.3 | 94.0±32.5 | 0.043 |
| iPTH (mg/dL) | 51.3±26.7 | 57.1±55.8 | 0.423 |
| Vitamin D (pg/dL) | 23.6±13.5 | 26.1±18.2 | 0.464 |
| TSH (IU) | 3.5±3.8 | 3.0±1.8 | 0.542 |
| Serum testosterone (ng/dL)* | 353.6±134.7 | 361.9±115.0 | 0.834 |
| Serum creatinine (mg/dL) | 1.1±0.3 | 1.1±0.2 | 0.670 |
| Fasting blood sugar (mg/dL) | 127.2±51.9 | 137.6±62.3 | 0.405 |
*in males only
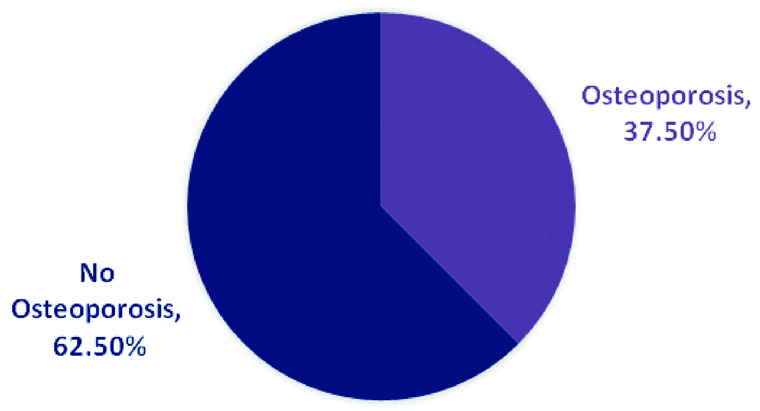
Correlation between T-score at distal radius and spine/hip is shown in [Table/Fig-3]. There was significant positive correlation with T-scores at spine (r=0.462, p<0.0001) and at hip (r=0.523, p<0.0001). T-scores at distal radius did not differ significantly by age and gender as evident from [Table/Fig-4]. As we observed osteoporosis in the unexpectedly greater proportion of patients at distal radius compared to other two sites, we performed further analysis to explore whether there can be a different cut-off than T-score <-2.5 for osteoporosis defined at distal radius. Compared to patients not having osteoporosis at spine/hip or combined, T-score at distal radius was found to be significantly higher in those with osteoporosis at spine (-4.1±1.7 vs -5.1±1.3, p<0.0001), hip (-4.3±1.7 vs -5.2±1.2, p<0.0001) and both sites combined (-4.1±1.7 vs -5.1±1.2, p<0.0001). Considering then cut-off of T-score ≤-5.0 at distal radius, we found osteoporosis prevalent in 37.5% (n=75) patients [Table/Fig-5]. However, there was no significant difference in the distribution of osteoporosis according to age groups (p=0.796) or gender (p=0.289) as shown in [Table/Fig-6].
Correlation of T-scores at radius with scores at spine and hip.
| Site | T-score distal radius |
|---|
| Pearson Correlation | p-value |
|---|
| Spine | 0.462 | <0.0001 |
| Hip | 0.523 | <0.0001 |
Distribution of distal radius T-score by different categories.
| Groups | T-score at distal radius | p-values |
|---|
| Age groups (years) | | |
| 51 to 60 | -4.3±1.5 | - |
| 61 to 70 | -4.5±1.7 | 1.000 |
| >70 | -4.7±1.8 | 0.989 |
| Gender | | |
| Male | -4.4±1.9 | 0.463 |
| Female | -4.6±1.4 |
| Osteoporosis at sites | | |
| Spine | Yes (n=67) | -5.1±1.3 | <0.0001 |
| No (n=133) | -4.1±1.7 |
| Hip | Yes (n=27) | -5.2±1.2 | <0.0001 |
| No (n=173) | -4.3±1.7 |
| Spine+Hip | Yes (n=71) | -5.1±1.2 | <0.0001 |
| No (n=129) | -4.1±1.7 |
Prevalence of osteoporosis defined by T-score ≤-5.0 at distal radius.
Percentage distribution of patients with cut-off of <-5.0 at distal radius by age and gender.
| Groups | T-Score ≤-5.0 (n=75) | T-Score >-5.0 (n=125) | p-value |
|---|
| Age groups (years) | | | |
| 51 to 60 | 19 (25.3) | 31 (24.8) | 0.796 |
| 61 to 70 | 44 (58.7) | 78 (62.4) |
| >70 | 12 (16.0) | 16 (12.8) |
| Gender | | | |
| Male | 35 (46.7) | 68 (54.4) | 0.289 |
| Female | 40 (53.3) | 57 (45.6) | |
Vitamin D deficiency was found in 91 (45.5%) patients. The proportion of patients with osteoporosis at distal radius considering T-score ≤-2.5 was non-significantly different (p=0.603) in patients with (46.1%) and without (53.9%) vitamin D deficiency as observed in [Table/Fig-7].
Vitamin D deficiency association with osteoporosis at distal radius by two cut-offs.
| Vitamin D Deficiency | T-score at distal radius (%) |
|---|
| ≤-2.5 (n=180) | >-2.5 (n=20) | p-value | ≤-5.0 (n=75) | >-5.0 (n=125) | p-value |
|---|
| Yes (n=91) | 83 (46.1) | 8 (40.0) | 0.603 | 36 (48.0) | 55 (44.0) | 0.582 |
| No (n=109) | 97 (53.9) | 12 (60.0) | 39 (52.0) | 70 (56.0) |
Discussion
Assessment of bone density using DXA at hip, spine or distal radius is the widely used technique for evaluation of osteoporosis. The international reference to define osteoporosis irrespective of the site is T-score of ≤-2.5 [6]. Osteoporosis is usually asymptomatic and is manifested after fractures at susceptible sites like hip, vertebra and distal radius. With increasing age, bone loss continues contributing to increased risk of fractures [10]. We have previously shown that in otherwise healthy T2DM patients, osteoporosis was found in 35% of patients when assessed at spine and hip [3].
Assessment of BMD at distal radius was associated with osteoporosis in the significant proportion of patients (90.0%) considering the T-score cut off of ≤-2.5. There were no differences in age, gender and in other biochemical parameters except for ALP which was significantly lower in patients with distal radius osteoporosis. This may be an incidental finding. Mean BMI was significantly lower in patients who had distal radial osteoporosis (p=0.048). Higher BMI has been reported to be associated with reduced risk of osteoporosis and fractures in T2DM at spine and hip [11]. On a similar note, a study by Anaforoglu I et al., in patients with radial/lumbar/hip osteoporosis reported significantly longer duration of diabetes and had a lower BMI [12].
As we observed osteoporosis in most of the patients at distal radius, we evaluated it further. There was a significant correlation of T-score at distal radius with that at the spine as well as hip. This is suggestive of the fact that bone loss occurs at all sites including cortical and trabecular bone of adults with T2DM aged 50 years and above. However, an interesting finding from Majima T et al., of reduced BMD at distal radius but not in spine or hip in T2DM adults suggested that bone metabolism may get affected differentially in cortical and cancellous bone [13]. We, therefore, analysed T-scores according to age, gender, and presence of osteoporosis at other sites. Though no significant differences in T-score of distal radius were noted according to age group, and gender categories, the mean scores were lower than reference standard of <-2.5. Further, significantly lower scores were noted in when assessed by presence or absence of osteoporosis at spine, hip or both sites combined. Therefore, considering cut-off of T-score ≤-5, we observed osteoporosis in 37.5% cases. A 10-years follow-up study in elderly women has shown that decreased BMD at distal radius is a strong risk factor for fracture [14]. But, there were no age and gender difference in the proportion of patients with radial osteoporosis considering this new cut-off value.
Vitamin D levels are known to affect bone metabolism. However, there was no association between vitamin D deficiency and osteoporosis defined by the standard or lower T-scores at distal radius. Similar to our finding, Perez-Diaz I et al., reported no significant relationship between vitamin D levels and osteoporotic fractures in postmenopausal women with T2DM [15]. Therefore, further investigation is warranted to understand the association of vitamin D levels with the radial bone loss in T2DM patients.
Our study brings out three important questions. First, whether bone loss occurs early at distal radius than at spine or hip? Previous reports also suggested a similar hypothesis [13,16]. Second, should the BMD cut-off to define osteoporosis be lower than the standard of T-score ≤-2.5. Third, what factors affect bone loss significantly at distal radius in comparison to that of the spine of hip?
Limitation
Limitations of the study include non-randomised design and non-inclusion control for comparison which would have provided greater insights in understanding the differences at distal radius. Being a cross-sectional study, it is difficult to determine whether the duration of T2DM correlated with osteoporosis, however a long-term, prospective study can identify such association in Indian population. Considering these, we further plan to study and understand the difference in osteoporotic fracture risk at these three sites.
Conclusion
T2DM affects bone loss and may increase the risk of osteoporotic fractures. Finding of a greater proportion of patients (90%) having osteoporosis at distal radius suggests that bone loss may occur early at the radial site than at hip and spine. No significant predictors of low BMD at distal radius demand careful evaluation of this site when assessing the risk of fractures. A bone-morphometric study in recently diagnosed T2DM evaluating the bone structure and loss at distal radius in comparison to the spine and hip would provide greater insights into understanding bone metabolism in otherwise healthy adult T2DM patients.
*in males only