Breast cancer is a major public health problem and the most common cause of cancer death among women throughout the world [1]. Palpable breast cancer is considered as a systemic disease with a local component and hence, multimodality treatment is mandatory. The treatment of breast cancer has been revolutionized by the understanding of the molecular basis of breast cancer. Oestrogen Receptor (ER), Progesterone Receptor (PgR) and Human Epidermal Growth Factor Receptor 2 (HER2/neu) expression are well known factors that predict the prognosis of breast cancer [2]. Advances in immunohistochemistry and molecular pathology studies have supplemented this by categorising breast cancer into different molecular subtypes with prognostic implications. In addition, the patterns of failure in breast cancer are very much driven by the intrinsic molecular subtypes, which have been studied mainly in early stage breast cancer [3,4]. Various studies have shown the relationship between the expression of different molecular markers and the aggressiveness of the disease as well as their response to chemotherapy. Compared to the west, in India more women present with locally advanced or metastatic disease [5]. The site of distant metastasis of breast cancer is predictive of clinical outcome [6].
We undertook this study to explore the relationship if any, between the different molecular subtypes and their predilection to a distinct distant metastatic site in locally advanced breast cancer.
Materials and Methods
This was a retrospective audit of the records of 70 women with a histopathological diagnosis of locally advanced breast cancer who received radiation therapy in the Department of Radiation Oncology, from January 2011 to September 2012. The case report forms of these patients were scrutinized and the demographic and treatment details were reviewed.
Locally Advanced Breast Cancer (LABC) includes a wide variety of clinical scenarios where a patient presents with advanced-stage non metastatic breast cancer. Any tumour that is greater than 5 cm or that involves the skin or chest wall, fixed axillary lymph nodes or ipsilateral supraclavicular, infraclavicular or internal mammary nodal involvement are included in this category [7]. Thus, all of stage III disease and a subset of stage IIB (T3N0) are considered as LABC and were included in the study. Among these, a subset of stage IIB (T3 N0) and a subset of stage IIIA (T3 N1) are considered operable LABC and the remaining subsets of IIIA, IIIB and IIIC disease are considered inoperable locally advanced breast cancer [8].
Immunohistochemical staining was performed on 4μ sections using ‘NovoLinkTM polymer detection system’ from Leica Biosystems Ltd., Newcastle upon Tyne, United Kingdom. Staining was performed using primary antibodies against Oestrogen receptor (ER, clone 6F11, Leica Biosystems), Progesterone receptor (PgR, clone 16, Leica Biosystems) and HER2/neu (clone CB11, Leica Biosystems). ER and PgR were reported as positive when ≥ 1% tumour cells showed nuclear positivity. HER2/neu was scored 0 when there was no staining or membrane staining in < 10% tumour cells; 1+ when faint or barely perceptible membrane staining was observed in > 10% tumour cells and the cells were stained in only part of their membrane; 2+ when weak to moderate complete membrane staining was observed in >10% tumour cells; 3+ when strong complete membrane staining was observed in >10% tumour cells. Score of 0 and 1+ were considered negative, 2+ as weakly positive and 3+ as strongly positive. Receptor status was taken from their Immunohistochemistry (IHC) reports for ER, PgR status and for those with HER2/neu 0 or 3+ on IHC, Fluorescent InSitu Hybridization (FISH) analysis was done for those with HER2/neu 1+ or 2+ on IHC.
Based on the receptor expression, we sub-stratified these patients into four basic molecular subtypes as luminal A (ER/PgR positive and HER2/neu negative), luminal B (ER, PgR and HER2/neu positive), triple negative (ER, PgR and HER2/neu negative) and HER2/neu enriched (ER and PgR negative, HER2/neu positive) [3]. We were not able to find the Ki67 index in most of the patients as it was not being done routinely during the time period considered.
Any recurrence of breast cancer beyond the ipsilateral or contralateral breast, chest wall or regional lymph nodes (including ipsilateral axillary, supraclavicular, or internal mammary lymph nodes) were considered as distant recurrence [3]. Time of first relapse, first site of relapse and sequence of development of metastasis in different sites, disease free survival and overall survival were documented. The patterns of distant failure observed in each molecular subtype were analysed.
Results
Between January 2011 and September 2012, 61.95% of women who presented with carcinoma breast to the unit had locally advanced or metastatic disease. We included 70 of these women with a histological diagnosis of carcinoma breast who had LABC at presentation. The demographic details of the patients are given in [Table/Fig-1]. Nine patients who developed metastasis and deteriorated during the course of treatment received palliative radiation to chest wall with concurrent Capecitabine, radiation to intact breast and spine and palliative chemotherapy. All patients with hormone receptor expression received hormonal therapy whereas only 2 out of the 8 HER2/neu positive patients received targeted therapy (Trastuzumab) due to financial constraints. Minimum follow-up was for 24 months with a range of 24 to 48 months.
Demographic profile of patients.
| Characteristic | Luminal A | Luminal B | Triple negative | HER2/neu enriched |
|---|
| Mean age in years (±SD) | 55.79±12.827 | 50±10.36 | 49.75±9.13 | 51.81±11.5 |
| Menopausal status |
| Pre | 12 (41.38%) | 5 (38.46%) | 8 (40%) | 4 (50%) |
| Post | 17 (58.62%) | 8 (61.54%) | 12 (60%) | 4 (50%) |
| Histology |
| IDCA | 26 (89.66%) | 11 (84.62%) | 17 (85%) | 8 (100%) |
| ILCA | 2 (6.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mixed and others | 1 (3.45%) | 2 (15.38%) | 3 (15%) | 0 (0%) |
| NACT | 7 (24.14%) | 5 (38.46%) | 8 (40%) | 5 (62.5%) |
| Surgery |
| MRM | 24 (82.76%) | 12 (92.31%) | 20 (100%) | 7 (87.5%) |
| BCS | 3 (10.34%) | 1 (7.69%) | 0 (0%) | 1 (12.5%) |
| No | 2 (6.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| EBRT |
| 50.4Gy/28F | 5 (17.24%) | 1 (7.69%) | 1 (5%) | 0 (0%) |
| 50Gy/25F | 20 (68.97%) | 12 (92.31%) | 17 (85%) | 8 (100%) |
| 42.5Gy/16F | 1 (3.45%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 35Gy/15F | 2 (6.9%) | 0 (0%) | 1 (5%) | 0 (0%) |
| 46Gy/23F | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) |
| No | 1 (3.45%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ACT | 21 (72.41%) | 11 (84.62%) | 15 (75%) | 6 (75%) |
IDCA: Invasive ductal carcinoma; ILCA: Invasive lobular carcinoma; NACT: NeoAdjuvant chemotharapy; MRM: Modified radical mastectomy; BCS: Breast conservation therapy; EBRT: External beam radiation therapy; ACT: Adjuvant chemotherapy
Among Luminal A subtype bone was the most common (47.06%) and the first site (in 70%) of distant failure followed by the brain in 23.53% patients. Liver was the most common site of distant recurrence (33.33%) and the first site too (in 66.66%) in luminal B patients. Triple negative tumours metastasized with equal frequency to the lung and bone (33.33%) with the lung being the first site of distant failure in 75% of patients. HER2/neu enriched subtype had lung as the commonest site of distant failure (40%) as also the first site of metastasis in 66.66% [Table/Fig-2,3 and 4]. In the present study by chi-square trend, the odds that a patient with luminal A, would metastasize to the bone was 2.66 times and to the brain was four times more than to the pleural cavity, which was the least likely site. The odds ratio for luminal A patients for bone being the first site of metastasis was 9.33, that for brain 8, lung 1.143 and pleural cavity 1 with respect to liver. The other molecular subtypes of locally advanced breast cancer did not show any significant trend on chi-square trend test. This can also be interpreted as a patient who metastasizes to the bone first is more likely (odds ratio 9.33) to be luminal A.
Distribution of failure events according to molecular subtypes.
| Molecular subtype | No. of patients | No. of pts. who failed | p-value | Local | Distant | No. of failure events | Local | Distant |
|---|
| Luminal A | 29 (41.4%) | 14 (%) | 0.54 | 4 | 10 | 21 | 4 | 17 |
| Luminal B | 13 (18.57) | 4 (%) | 1 | 3 | 10 | 1 | 9 |
| Her 2 neu enriched | 8 (11.4%) | 6 (%) | 3 | 3 | 8 | 3 | 5 |
| Triple negative | 20 (28.57%) | 6 (%) | 2 | 4 | 11 | 2 | 9 |
Distribution according to the site of distant metastasis among the molecular subtypes.
| Site of metastatic event | Luminal A | Luminal B | Triple negative | HER2/neu enriched | p-value |
|---|
| Bone | 8 (47.06% of events) | 2 (22.22%) | 3 (33.33%) | 1 (20%) | 0.707 |
| Liver | 2 (11.76%) | 3 (33.33%) | 1 (11.11%) | 1 (20%) |
| Lung | 2 (11.76%) | 2 (22.22%) | 3 (33.33%) | 2 (40%) |
| Pleural effusion | 1 (5.88%) | 1 (11.11%) | 0 (0%) | 1 (20%) |
| Brain | 4 (23.53%) | 1 (11.11%) | 1 (11.11%) | 0 (0%) |
| Subcutaneous | 0 (0%) | 0 (0%) | 1 (11.11%) | 0 (0%) |
Distribution according to first distant event among the molecular subtypes.
| Site of 1st recurrence | Luminal A | Luminal B | Triple negative | HER2/neu enriched | p-value |
|---|
| Bone | 7 (70%) | 1 (33.33%) | 2 (50%) | 0 (0%) | 0.328 |
| Liver | 1(10%) | 2 (66.66%) | 1 (25%) | 1 (33.33%) |
| Lung | 2 (20%) | 2 (66.66%) | 3 (75%) | 2 (66.66%) |
| Pleural effusion | 0 (0%) | 1 (33.33%) | 0 (0%) | 1 (33.33%) |
| Brain | 2 (20%) | 0 (0%) | 1 (25%) | 0 (0%) |
| Subcutaneous | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
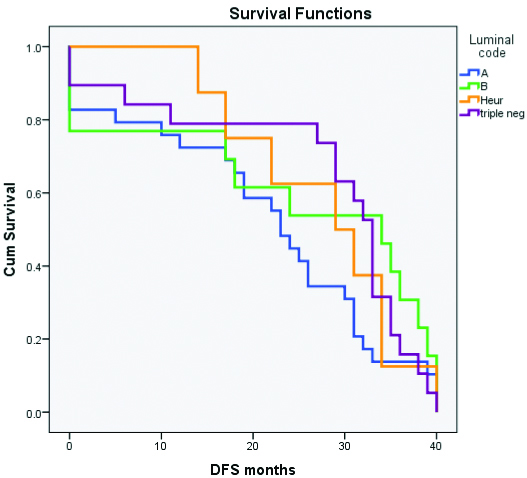
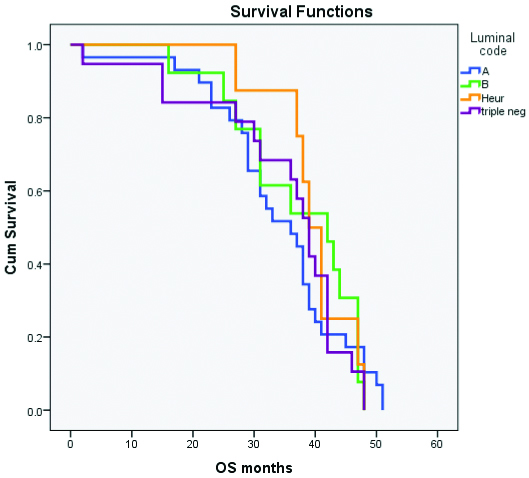
The mean time to first recurrence (DFS) was shortest in luminal A (20.17 months) and the longest in HER2/neu enriched (27.63 months). The mean overall survival too showed a similar trend with 34.28 months for luminal A and 39.75 months for HER2/neu enriched subtype [Table/Fig-5,6 and 7]. But the variations in survival were not statistically significant. The median survival after the development of metastasis was 21 months for luminal A and 25 months for luminal B whereas it was 8.5 and 7 months in triple negative and HER2/neu enriched groups respectively which did not attain statistical significance. But, median survival after the development of metastasis was longer among luminal subtypes than among the non luminal subtypes. (p=0.056) [Table/Fig-8].
Survival data of various molecular subtypes.
| Molecular subtype | Mean time to first recurrence in months (±SD) | Median DFS in months | Median OS(±SD) in months | Median survival after metastasis (±SD) in months |
|---|
| Luminal A | 21.24±13.14 | 23 | 36±11.12 | 21±19.4 |
| Luminal B | 24.69±16.09 | 34 | 42±10.36 | 25±13.75 |
| Triple negative | 27.40±12.54 | 32.5 | 38.5±11.98 | 8.5±12.29 |
| HER2/neu enriched | 27.63±9.09 | 30 | 40±6.52 | 7±6.51 |
| p-value | 0.239 | 0.239 | 0.579 | 0.231 |
DFS: Disease free survival; OS: Overall survival
Kaplan Meier curve for disease free survival.
Kaplan Meier curve for overall survival.
Difference in median survival after metastasis between luminal and non luminal subtypes.
| Median survival after metastasis | Luminal subtypes (IQR) | Non luminal subtypes (IQR) | p-value |
|---|
| In months | 25 (7-41) | 7 (1-15) | 0.056 |
Discussion
The concept of molecular subtypes of breast cancer was introduced by Perou CM et al., in their paper titled “Molecular portraits of human breast tumours” in the year 2000 and they brought out an intrinsic gene list [9]. Several studies have shown the relation between the expression of different molecular markers and their aggressiveness [10,11] as well as their response to chemotherapy [11-13] and radiotherapy [14]. The preferential relapse of molecular subtypes in different metastatic sites has been studied using this intrinsic gene list mainly in early stage breast cancer [15]. Such studies are lacking in locally advanced breast cancer as the number of patients presenting in advanced stages of the disease are relatively less in the west.
An immunohistochemical surrogate panel supported by gene expression profile studies [16,17] used by Kennecke H et al., divides breast cancers into five molecular subtypes. Luminal A (ER positive and/or PgR positive and Ki-67<14%), luminal B (ER positive and/or PgR positive and Ki-67 >14%), luminal/HER2 (ER positive and/or PgR positive and HER2/neu positive), HER2/neu enriched (ER negative and PgR negative and HER2/neu positive), and basal-like (ER negative and PgR negative and HER2/neu negative) [4]. This study on a large cohort of 3726 patients with early stage breast cancer established that the different molecular subtypes of breast cancer show distinct pattern of metastasis and significant differences in survival after relapse as far as early stage breast cancer is concerned. Brain metastasis was found to be more frequent in non-luminal subtypes with a significantly lower incidence in luminal subtypes. Luminal subtypes metastasized preferentially to the bone which was the least common site among basal type breast cancers. The rate of development of liver metastasis was also significantly low among basal type which showed high rates of relapse in brain, lung and distant lymph nodes. HER2/neu positive tumours showed high rates of metastasis to brain, liver and lung.
Smid M et al., studied the preferential sites of relapse of molecular subtypes of breast cancer and the molecular pathways involved [15]. Three percentage of their study subjects were locally advanced breast cancer patients. They noted that preferential bone relapse was found only in luminal A among the luminal subtypes. Lung metastases were more common in basal subtype and least in luminal A. Liver was more commonly involved in Her 2-neu enriched group and least among luminal B patients. Most of the brain metastasis was among basal type tumours. They were able to show that shared biology and gene expression patterns are present between the molecular subtypes and their preferred sites of metastasis. The association of “focal adhesion” pathway in lung metastasis and that of “WNT signaling” pathway in bone and brain metastasis was found to be significant.
The patterns of distant recurrence in molecular subtypes of breast cancer were studied among Korean women by Park HS et al., [3]. They included both early stage and locally advanced breast cancer. In their study, bone metastasis was the commonest first site of metastasis in luminal subtypes whereas in HER2/neu enriched and triple negative breast cancer sites other than the bone and brain were the commonest first sites of relapse. Brain metastasis was uncommon in HER2/neu enriched type but was found in all the other subtypes. No significant difference was found in the cumulative frequency of other metastatic sites between the subtypes or in the distant relapse free survival among the molecular subtypes but, overall survival in patients who develop recurrence more than 24 months after surgery was better among luminal subtypes.
In the present study conformed to earlier reports on early stage breast cancer 4 with luminal A being the most common subtype in locally advanced breast cancer and HER2/neu enriched the least common. But unlike early stage disease, triple negative tumours were more common than luminal B subtype. We did not perform immunohistochemical testing after NACT and surgery to look for the changes in hormone receptor expression which occurs in 21% of patients.
In the present study, the lung was the commonest site of first relapse in all subtypes except luminal A where the bone was the first site of relapse. Preferential metastasis to bone was seen in luminal A and not luminal B which is in accordance to what has been reported with early stage disease [15]. Studies which included only early stage breast cancer have shown lower incidence of brain metastasis in luminal subtypes and higher incidence in HER2/neu enriched disease [4,15]. Contrary to this, we found brain metastasis in all subtypes except HER2/neu enriched subtype. This may be a unique pattern seen in LABC due to the short overall survival as has been reported by Park HS et al 3, on a cohort of patients with early stage and locally advanced breast cancer. It can also be explained by the fact that most of our patients with HER2/neu enriched tumours did not receive the benefit of targeted therapy due to financial constraints, losing out on the survival benefit. The incidence of pleural metastasis was highest in HER2/neu enriched and was not seen in triple negative breast cancer.
Among all the molecular subtypes of LABC, we found that the most common site of metastasis was the most common first site of distant relapse as well. In luminal A it was the bone, in luminal B the liver, in triple negative and HER2/neu enriched it was the lung. A trend towards more odds of developing bone and brain metastasis and metastasizing first to the bone and then brain were noted only in luminal A by chi-square testing. Such a trend could not be established in the other molecular subtypes.
Significant difference was not noted among the various molecular subtypes as far as mean Overall Survival (OS) and Disease Free Survival (DFS) is concerned. This is in accordance with the results of Park HS et al., where there was no significant variation in DFS and OS between the molecular subtypes in patients developing relapse within 24 months of treatment [3]. But median survival after the development of metastasis was longer among luminal subtypes than among the non-luminal subtypes (p=0.056).
Limitation
Our study was limited by being a retrospective audit with a small sample size and is not powered to draw conclusions. Not all patients with LABC received neoadjuvant chemotherapy and the change in receptor expression after neoadjuvant chemotherapy was not considered. But, as only two of the patients in our study received targeted therapy, it is less likely that the natural history of HER2/neu enriched disease is altered as they received systemic treatment similar to other molecular subtypes.
Conclusion
Our study revealed luminal A subtype to be the commonest molecular subtype of locally advanced breast cancer. In all the molecular subtypes, the commonest site of metastasis is the commonest first site of distant relapse as well. In luminal A it is the bone, in luminal B the liver and in triple negative and HER2/neu enriched it is the lung though the odds ratio was significant only among luminal A patients. There is no significant difference in DFS and OS between the molecular subtypes in our study population. But, luminal subtypes have a longer survival after the development of metastasis. There is need for a well-structured prospective study protocol with a longer follow-up with a larger number of patients before tailor made surveillance protocols can be developed for the various molecular subtypes of locally advanced breast cancer.
IDCA: Invasive ductal carcinoma; ILCA: Invasive lobular carcinoma; NACT: NeoAdjuvant chemotharapy; MRM: Modified radical mastectomy; BCS: Breast conservation therapy; EBRT: External beam radiation therapy; ACT: Adjuvant chemotherapy
DFS: Disease free survival; OS: Overall survival