The incidence of thyroid nodules are increasing steadily, of which most nodules are benign [1-3]. HRUS is a sensitive imaging test that is used for the examination of the thyroid gland for detection of thyroid lesions, to calculate their dimensions accurately, identifying their internal structure, vascularisation, and evaluation of the diffuse changes in the thyroid parenchyma. HRUS of the thyroid can be used as a confirmation test to find the presence of a thyroid nodule when the physical examination is found to be equivocal between the thyroid nodules and cervical masses from other origins, like cystic hygroma, thyroglossal duct cyst, and lymphadenopathy.
Ultrasonography (US) is relatively cheap, easily accessible, rapidly performed and has an advantage of no exposure to ionising radiation. Since the thyroid gland is superficially located, HRUS can demonstrate normal thyroid anatomy and pathologic conditions with remarkable clarity. As a result, this technique has come to play an increasingly important role in the diagnostic evaluation of thyroid diseases. Several sonographic patterns are useful for predicting thyroid malignancy and some of these patterns have high sensitivity and specificity [4-6].
Sonoelastography (SE) is a relatively new non-invasive technique for imaging stiffness or elasticity of the tissue by measuring movement or deformation of tissue in response to a small applied pressure. It is basically a “virtual palpation” of the lesion. SE is used to quantify the hardness of a thyroid lesion in relation to the surrounding tissue, is useful in differentiating between benign and malignant tissue [7,8].
Real-time SE is a newly developed diagnostic tool that evaluates the degree of distortion of the ultrasonography beam while an external force is applied. It is based on the principle that the softer parts of the tissue deform more easily than the harder parts under compression. Thus, a semi-quantitative determination of tissue elasticity is observed [9].
The strain ratio is a semi-quantitative analysis that compares the stiffness or strain of two different areas within the same image: two Regions Of Interest (ROIs) are manually applied on the screen, one on the target lesion and the second on the reference normal thyroid, allowing the calculation of their strain ratio by the immediate real-time US machine analysis [10].
There are several limitations to SE which include histology of the nodule, intra and interobserver variability and lack of standardisation. This is an examiner dependent method. The present prospective study was performed to assess the diagnostic performance of conventional ultrasonography, colour mapping and strain ratio using SE for characterising thyroid nodules.
Materials and Methods
A prospective study was conducted in which 65 patients were examined from October 2015-August 2017. All age group patients who were referred for thyroid US and were detected to have a thyroid nodule which was subsequently confirmed by FNA/histopathology were included in the study. Those patients who refused FNAC and whose final confirmatory diagnosis could not be established were excluded from the study. Lesions with more than 50% cystic degeneration/calcification and with a calcific shell were excluded. Cases with histologic findings of chronic inflammation were also excluded. Ethical approval for this study was granted by the Medical Research Ethics Committee of Kasturba Medical College, Mangalore, and consent was obtained from all the patients.
Conventional thyroid sonography and sonoelastography
Thyroid sonography and sonoelastography were performed using LOGIQ S7 Expert/Pro (General Electrical Co. USA) fitted with 6-15 MHz matrix linear array transducer. At first, conventional sonography was performed on each nodule to assess size, echogenicity, presence, and type of calcification, margins, anteroposterior/transverse diameter ratio of 1 or greater, and Doppler colour flow pattern of the thyroid nodules [4,11]. Sonoelastographic measurements were performed after conventional sonography. The probe was placed on the neck. The light and cyclic probe pressure have to be harmonic with a constant rate of displacement [12], and a box large enough to include the entire nodule and the surrounding thyroid and parathyroid tissue was highlighted by the operator, for calculating the strain images without noise. The device shows a colour-coded bar ranging from red to green to evaluate examination quality. The green colour was required to enable a good evaluation. The elastogram was displayed over the B-mode image in a colour scale ranging from red, indicating components with the greatest elastic strain (i.e., softest components), to blue, indicating components with no strain (i.e., hardest components). Visualisation patterns of the nodules on the elastograms were classified into four types as shown in [Table/Fig-1], where pattern four was assumed to be the characteristic findings of malignancy.
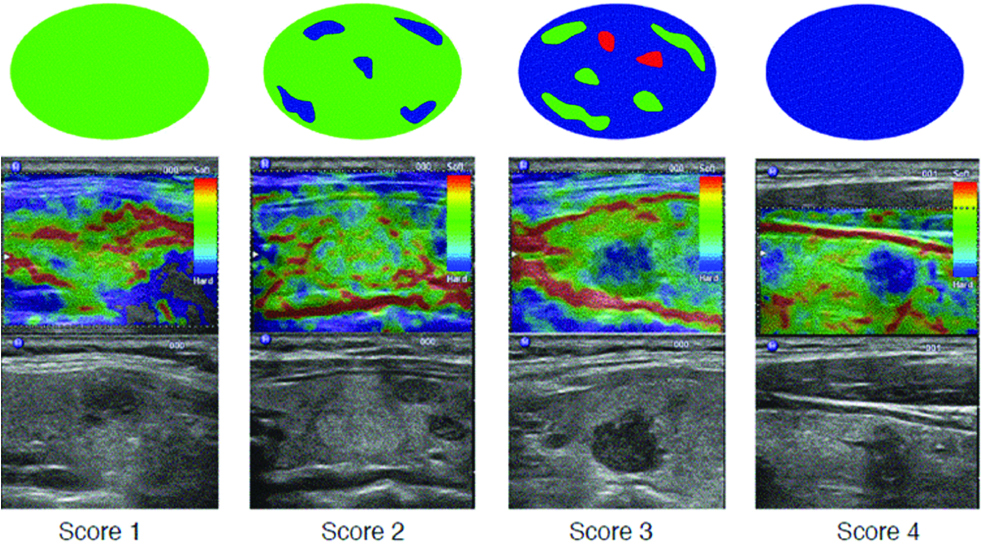
The mean strain index of the thyroid nodule and that of the surrounding thyroid tissue were measured. The surrounding tissue was selected as a reference at the same depth as the nodule. The average strain of the lesion was determined by selecting a representative region of interest from the lesion and adjacent thyroid tissue; the resultant strain ratio was automatically calculated by the machine. A strain ratio of >2.8 was set as the predictor of nodule malignancy. This cut-off point was decided by a ROC curve so that sum of sensitivity and specificity was maximised.
Fine-needle aspiration cytology and histopathological evaluation
All nodules underwent US-guided FNAC, after obtaining the patient’s informed consent. FNAC was performed with a 23 G needle attached to a 10 mL syringe; the material collected and prepared with the Thin-prep method was sent to the medical pathologist together with a statement containing the patient’s data, the clinical question, the characteristics of the aspirate, and the number of samples taken for each nodule. Histopathological Evaluation (HPE) was done wherever possible. Cytological results were reported according to the Bethesda classification, which is a uniform reporting system for thyroid FNA results [13]. The six general diagnostic categories included are: 1) non-diagnostic or unsatisfactory; II) benign; III) atypical of undetermined significance; IV) follicular neoplasm or suspicious for a follicular neoplasm; (V) suspicious for malignancy; or (VI) malignant.
Statistical Analysis
Statistical analysis was conducted using package SPSS version 17.0 for windows. Test of significance was assessed using the chi-square test. p<0.05 was considered significant. The diagnostic test was calculated using sensitivity, specificity, negative predictive value, positive predictive value and accuracy rate.
Results
A total of 65 patients were examined. FNA/HPE revealed 14 (21.5%) malignant (six papillary carcinomas, three poorly differentiated carcinoma, two anaplastic carcinomas and three follicular carcinomas) and 51 (78.5%) benign nodules. Out of these 14 malignant nodules, 13 (92.9%) cases were found in patients above 50 years and only 1 (7.1%) case was found below 50 years. No incidence of the malignant lesion was seen below the age of 30 years in the present study [Table/Fig-2]. The sonographic patterns indicating malignancy include nodule hypoechogenicity [Table/Fig-3] seen in 71.4% of malignant lesions, nodule Anteroposterior(AP)/Transverse (TR) diameter >1 [Table/Fig-4] seen in 35.7% of malignant lesion, intra-nodular blood flow pattern seen in 92.9% of malignant lesions [Table/Fig-5] and microcalcification was seen in 64.3% of malignant lesions [Table/Fig-6].
Correlation of age with FNA/HPE.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Age | 30 and below | 0 | 7 | 7 |
| 0% | 100.0% | 100.0% |
| 0% | 13.7% | 10.8% |
| 31-40 | 1 | 11 | 12 |
| 8.3% | 91.7% | 100.0% |
| 7.1% | 21.6% | 18.5% |
| 41-50 | 0 | 12 | 12 |
| 0% | 100.0% | 100.0% |
| 0% | 23.5% | 18.5% |
| 51-60 | 8 | 11 | 19 |
| 42.1% | 57.9% | 100.0% |
| 57.1% | 21.6% | 29.2% |
| 61-70 | 3 | 8 | 11 |
| 27.3% | 72.7% | 100.0% |
| 21.4% | 15.7% | 16.9% |
| Above 70 | 2 | 2 | 4 |
| 50.0% | 50.0% | 100.0% |
| 14.3% | 3.9% | 6.2% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Comparison with FNA/HPE and echogenicity.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Gray scale findings (APxTR) | Hyperechoic | 0 | 33 | 33 |
| 0% | 100.0% | 100.0% |
| 0% | 64.7% | 50.8% |
| Hypoechoic | 10 | 0 | 10 |
| 100.0% | 0% | 100.0% |
| 71.4% | 0% | 15.4% |
| Isoechoic | 0 | 2 | 2 |
| 0% | 100.0% | 100.0% |
| 0% | 3.9% | 3.1% |
| Mixed | 4 | 16 | 20 |
| 20.0% | 80.0% | 100.0% |
| 28.6% | 31.4% | 30.8% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Comparison with FNA/HPE and nodule dimensions (AP/TR>1).
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Gray scale findings (APxTR) | M | 5 | 2 | 7 |
| 71.4% | 28.6% | 100.0% |
| 35.7% | 3.9% | 10.8% |
| B | 9 | 49 | 58 |
| 15.5% | 84.5% | 100.0% |
| 64.3% | 96.1% | 89.2% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Comparison between FNA/HPE and vascularity.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Colour doppler | Internal | 13 | 0 | 13 |
| 100.0% | 0% | 100.0% |
| 92.9% | 0% | 20.0% |
| No vascularity | 0 | 6 | 6 |
| 0% | 100.0% | 100.0% |
| 0% | 11.8% | 9.2% |
| Peripheral | 0 | 28 | 28 |
| 0% | 100.0% | 100.0% |
| 0% | 54.9% | 43.1% |
| Peripheral and internal vascularity | 1 | 17 | 18 |
| 5.6% | 94.4% | 100.0% |
| 7.1% | 33.3% | 27.7% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Comparison between FNA/HPE and calcification.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Calcification | Macro | 2 | 5 | 7 |
| 28.6% | 71.4% | 100.0% |
| 14.3% | 9.8% | 10.8% |
| Micro | 9 | 0 | 9 |
| 100.0% | .0% | 100.0% |
| 64.3% | .0% | 13.8% |
| No | 3 | 46 | 49 |
| 6.1% | 93.9% | 100.0% |
| 21.4% | 90.2% | 75.4% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
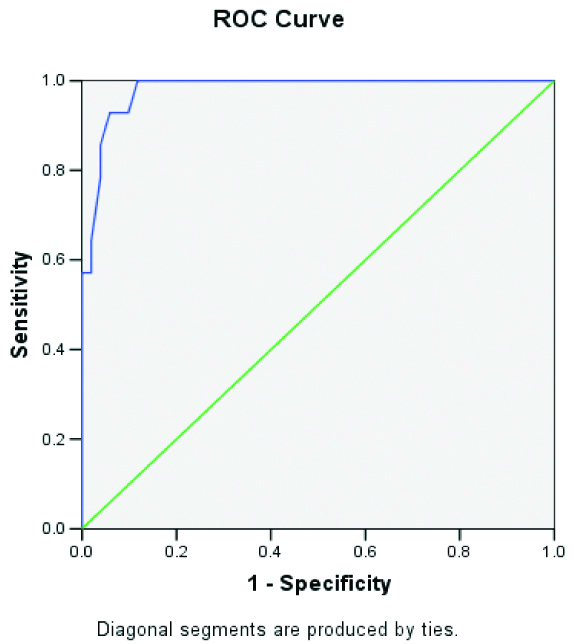
The strain ratio was calculated between the thyroid nodule and surrounding thyroid tissue at the same depth. There were significant differences between the strain ratios for benign and malignant lesions (p<.001). The ultrasound elastography score is indicating malignancy in 81.3% of malignant nodules [Table/Fig-7]. [Table/Fig-8] shows the ROC curve for the strain ratio assessment method used for differentiating malignant from benign lesions. The AUC was 0.98 and the best cut-off value was 2.8 with sensitivity, specificity, PPV, NPV and overall accuracy of 92.86, 94.12, 81.25, 97.96 and 93.85 respectively.
Comparison with FNA/HPE and strain ratio.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Strain Ratio | M(>2.8) | 13 | 3 | 16 |
| 81.3% | 18.8% | 100.0% |
| 92.9% | 5.9% | 24.6% |
| B | 1 | 48 | 49 |
| 2.0% | 98.0% | 100.0% |
| 7.1% | 94.1% | 75.4% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Area under the curve with respective values for sensitivity, specificity, PPV, NPV and overall accuracy.
 |
| | Confidence interval |
| | Lower | Upper |
| Sensitivity | 92.86 | 79.37 | 106.35 |
| Specificity | 94.12 | 87.66 | 100.58 |
| PPV | 81.25 | 62.12 | 100.38 |
| NPV | 97.96 | 94.00 | 101.92 |
| Overall accuracy** | 93.85 | 88.00 | 99.69 |
p<0.001, HS
Discussion
A thyroid nodule is defined as a discrete lesion within the thyroid gland that is distinguishable from the adjacent parenchyma at US [14]. With respect to nodule characterisation and pattern recognition, authors divided the nodules according to echogenicity, AP/TR ratio >1, calcifications within these nodules and the vascularity of these nodules. According to Kim MJ et al., a hypoechoic nodule is the one that is hypoechoic to the strap muscles anterior to the thyroid gland [15].
Coming to the patterns of echogenicity of the nodules included in the present study, out of the 14 cases that were found to be malignant on FNA/HPE, 10 (71.4%) lesions were hypoechoic and 4 (28.6%) were mixed echoic lesions. There were no cases in which malignancy was found in a hyperechoic lesion.
To conclude, malignant lesions were largely found to be hypoechoic and benign lesions were predominantly hyperechoic. The present study concurs with that of Moon WJ et al., who studied various benign nodules on US and their findings showed that benign nodules were either isoechoic or hyperechoic (sensitivity, 57%, specificity, 88%) and had a spongiform appearance (sensitivity, 10%, specificity, 100%) [16].
A study done by Papiniet et al., showed that malignant nodules typically appear solid and hypoechoic compared to normal thyroid parenchyma. This combination shows 87% sensitivity when detected for thyroid malignancy but also shows a low specificity of 15.6%-27% and a low positive predictive value. This feature can also be seen in 55% of benign nodules. When a thyroid nodule is seen to be hypoechoic with a darker appearance than the infrahyoid or strap muscles of the neck, the specificity of the same is increased to 94% while sensitivity is reduced to 12%. The presence of hypoechogenicity highly suggests malignancy [4].
Next moving onto calcification within the nodules, out of the 65 cases, 16 (24.6%) cases showed calcification of which 7 (43.7%) had macro-calcification and 9 (56.3%) had micro-calcification. Out of the seven cases with macro-calcification, in the present study, 5 (71.4%) nodules were found to be benign on FNA/HPE. There were nine cases of microcalcification of which, 9 (100%) were found to be malignant. Hence, there was a strong association between micro-calcification and malignancy in the present findings.
Micro-calcifications represent psammoma bodies, which are round, laminar calcific deposits [17]. They appear as punctate echogenic foci <1 mm in size and without acoustic shadowing. In contrast, macro-calcifications are coarse calcifications >1 mm in size and may demonstrate acoustic shadowing [18]. Micro-calcifications are typical of papillary thyroid carcinoma, whereas macro-calcifications (including rim calcifications) are less ominous but may be seen in both medullary and papillary carcinoma [19].
In the present study 28 (43.1%) cases showed peripheral vascularity and both peripheral and internal vascularity was seen in 18 (27.7%) cases. Out of these 28 cases with peripheral vascularity, all 28 (100%) nodules were found to be benign on FNA/HPE. Out of the 18 cases with both peripheral and internal vascularity, 17 (94.4%) were benign and 1 (5.6%) was malignant on FNA/HPE. Out of the 13 cases with internal vascularity alone, in the present study, all 13 (100%) nodules were found to be malignant on FNA/HPE. Chan BK et al., studied hyper vascularity within the central aspect of a nodule and found that suggestive of a malignant process than peripheral hypervascularity [20].
In the present study, the nodules were studied with strain ratio criteria [9]. When a score of four in the Ueno et al., criteria were considered to be malignant, 12 cases were found to be malignant, of which 10 (83.3%) proved to be malignant on FNA/HPE. On the other hand, 53 cases were deemed to be benign, of which 49 (92.5%) were found to be benign on FNA/HPE. The resultant p-value (p<0.001) was highly significant [Table/Fig-9]. Coming to the strain ratio, with a value >2.8 considered to be malignant; 14 cases were found to be malignant, of which 12 (85.7%) proved to be malignant and 2 (14.3%), benign on FNA/HPE. Of the 49 cases that were found to be benign on strain ratio, 48 (98%) were found to be benign and 1 (2%) found to be malignant on FNA/HPE.
Comparison with FNA/HPE and Ueno/Asteria criteria.
| FNAC/HPE findings | Total |
|---|
| M | B |
|---|
| Elastography score | M(4) | 10 | 2 | 12 |
| 83.3% | 16.7% | 100.0% |
| 71.4% | 3.9% | 18.5% |
| B | 4 | 49 | 53 |
| 7.5% | 92.5% | 100.0% |
| 28.6% | 96.1% | 81.5% |
| Total | 14 | 51 | 65 |
| 21.5% | 78.5% | 100.0% |
| 100.0% | 100.0% | 100.0% |
Chong Y et al., found that although US elastography is helpful to predict malignant thyroid nodules, the addition of strain ratio to colour mapping does not improve performance compared to colour mapping alone. It was also identified that the best cut-off value for predicting a malignancy is a ratio higher than 3.1 [21]. However, it was seen that compared to any other US features, strain ratio performed slightly better than qualitative USE according to the meta-analysis did in 2013 [22].
Another issue is the effect of different technical parameters in the evaluation of strain ratio. A study done by Havre RF et al., on a US phantom suggested that the strain ratio only depends on the position of the ROI to the reference area and does not depend on the size or the dynamic range [23]. This provides a better reproducibility as compared to the qualitative scoring that is prompted by the preselected elasticity dynamic range. Therefore, care should be taken to always position the reference ROI in normal parenchyma in the same depth as the examined nodule, even though the ROI may be very small due to limited normal thyroid. In cases of the multinodular thyroid with no normal parenchyma, the reference ROI can be positioned in the contralateral lobe but always in the same depth.
The histological features of the nodules, as well as its inherent technical limitations, can lead to pitfalls. A confounding factor that can be seen in elasticity imaging can be fibrosis. Although there are many studies that can prove the correlation between the different levels of stiffness and fibrosis, there is no study that can evaluate the presence of fibrosis in thyroid nodules using SE [24]. SE results are not influenced by the presence of autoimmune thyroiditis. However, calcifications, the presence of partially cystic or colloid components, the location of the isthmus, size of nodule and presence of a multinodular goitre can be related to increased levels of stiffness [25,26]. False negative results may be seen in cases of follicular carcinomas because they are soft and therefore can be easily missed with SE. However, in the present study follicular carcinomas are seen to have high strain ratio and appear hard on elastography colour maps.
Limitation
The limitation of this study was it was operator dependent and has inter and intraobserver variability based on the pressure used for compression. This has been tried to overcome using a colour coded indicator to achieve optimal compression.
Conclusion
Being a safe, simple and repeatable test without radiation exposure to the patient, it is worthy of being included in the routine diagnostic workup. Elastography with ultrasonography is a quick and easy method of obtaining additional information to differentiate benign versus malignant aetiology when assessing thyroid nodules. It also helps in the choice of the nodule (s) for FNA/BIOPSY and reduces the number of FNA procedures.
p<0.001, HS