Acrylamide is a water-soluble, vinyl monomer widely used in manufacture of polymers for use in the mining, paper, water purification, and oil field industries. Monomeric acrylamide exposure results in a characteristic peripheral neuropathy, with the weakness of the limbs, especially hind limbs, in animals and humans [1,2]. In the year 2002, the Swedish Food Administration stated the presence of acrylamide in heat-treated food products [3]. The formation of acrylamide is linked with high-temperature (higher than 200°C) cooking process in some high carbohydrate-rich foods, especially when asparagine reacts with sugar [4].
Acrylamide affects various reproductive parameters in mice which include increased abnormal sperm morphology [5], testicular damage including vacuolations and swelling of the round spermatid [6], and DNA damage during specific germ cell stages [7]. Male rats treated with acrylamide showed a significant reduction in mating, fertility, and transport of sperm in the uterus [8], suggesting that acrylamide exerts reproductive toxicity in male rodents [9].
A study by Wang H et al., experimented acrylamide toxicity in male rats which demonstrates retarded growth, a significant decrease in epididymal sperm reserves and the presence of histopathological lesions in the testis of treated rats [10]. In a recent study conducted by Rajeh N et al., acrylamide at the dose level of 45mg/Kg has been reported to show the reduction in luminal sperm reserve, shedding of normal epithelium in the lumen of the seminiferous tubule, maturation arrest in some tubules and multinucleated giant cells with vacuolations in between inner cells of the tubules [11].
In recent years, intake of high-carbohydrate rich food, which is cooked at high temperatures is very common among people which may lead to the exposure to high doses of acrylamide. The reviews also suggest that acrylamide results in reproductive toxicity in male. But there is a lack of supporting data in the studies reported with respect to histopathological abnormalities. Hence, the present study was designed to assess the effect of acrylamide on reproductive organs with a special focus on histopathological changes with other parameters including body weight, wet testes and epididymal weight, sperm count, motility and morphology and histological changes.
Materials and Methods
This is an animal experimental study consisting of 18 adult male albino Wistar rats of age 45 to 55 days with weight 165 to 175g for a duration of one year from November 2015 to November 2016. The ethical clearance for the animal-based procedures was obtained from the Institutional Animal Ethical Committee (IAEC), KMC Manipal, Manipal University, Karnataka, India. (IAEC/KMC/94/2015 dated 20.11.2015). Animals were maintained as per the National guidelines protocol under standard environmental conditions. Polypropylene cages with the bedding of paddy husk were used for animal housing. The animals were maintained at controlled temperature and humidity for 21 days under constant 12-hour light/12-hour dark schedule. Animals were fed with clean drinking water and standard rat pellet diet ad libitum.
Dose Selection and Treatment: Acrylamide 99% pure (extra pure) was obtained from Sisco Research Lab Pvt., Ltd., Taloja Maharastra and Potassium Dihydrogen Phosphate was obtained from Glaxo lab (99.5-100.5%), Mumbai, India. The range of dose of acrylamide was decided based on the data on occupational exposure to the human population. The dose calculation was done using the formula that suggestively converses chemical exposure to several species by considering body surface area and metabolism [12].
Human Equivalent Dose (mg/kg = Animal NOAEL mg/kg) × (Weightanimal {kg}/Weight human {kg})(1-0.67)
Study Design: According to the dose administered, the animals were divided into three-control, low exposure and high exposure groups. The control group were given distilled water (dose = 0 mg/kg), the low dose group was with 6.25 mg/kg body weight/day and the high dose given was 25 mg/kg body weight/day. There were six rats in each group with their average body weight equalized in all the groups before the initial exposure. The acrylamide with different doses was administered for 21 consecutive days by oral gavage by preparing fresh solutions with distilled water according to the weight of rats.
During the treatment period, the body weight of each rat was recorded daily before the administration of acrylamide. As the two animals in high dose group were dead on 17th day of exposure and the following days the other rats were found weak with neurological disturbances, we terminated the treatment on 21st day.
At the end of treatment, the rats were sacrificed under ether anaesthesia, wet weights of testis and epididymis were noted, cauda epididymal sperm count and motility were determined, and sperm morphology was examined. The left testis and left epididymis were fixed overnight in Bouin’s fluid followed by routine histologic processing and embedding in paraffin wax. Right cauda epididymis was utilized to prepare sperm suspension for sperm count, sperm motility and sperm morphological analysis.
Epididymal Sperm Count: After weighing the whole right epididymis, it was minced thoroughly in 3000 μL of phosphate buffered saline. Twenty μL of this fluid was diluted with 380 μL of sperm diluting fluid (5% sodium bicarbonate solution). Ten μL of this solution was charged into improved Neubauer counting chamber using a micro pipette. Sperms were allowed to settle into the squares for 2 minutes. Then, sperms in the four large corner squares (WBC squares) were counted under high power objective (40× magnification) of Leica compound microscope. The epididymis sperm count was then determined using the formula: Total number of sperms = Number of sperms counted × depth factor (10) × dilution factor (6000)/area counted (4 mm2). The total number of sperms obtained from the calculation were expressed as a number of sperms per epididymis [13].
Epididymal Sperm Motility: The Epididymal sperm motility was determined using improved Neubauer haemocytometer. A small quantity of epididymis sperm suspension was charged into the improved Neubauer counting chamber using a micropipette. The haemocytometer was then placed under the high-power objective (40× magnification), and motility of the sperms was analysed. The sperms were then categorized into two broad categories of motility: Motile sperms and non-motile sperms. Motile sperms have been subcategorized into actively motile and sluggishly motile. If the sperms were exhibiting unidirectional or multidirectional movement, it was categorized into actively motile sperms, and the sperms were exhibiting slightly motile without any progression were classified into sluggishly motile sperms. Those sperms which displayed no movement at all, were categorized into non-motile. Hundred sperms were assessed for motility and percentage of motile (active and sluggish), and non-motile sperms were estimated.
Epididymal Sperm Morphology: Thin, dry smears were prepared from the epididymal sperm suspension using a slide on slide smearing technique and dried at room temperature. The dried smears were then stained using Leishman’s staining technique. The smears were treated with Leishman’s stain for 2-3 minutes (care was taken to make sure the stain did not dry), then twice the volume of 6.8 pH phosphate buffer was added and mixed thoroughly. The buffer was allowed to act for 8-10 minutes and the end of 10 minutes’ excess stain was washed off using tap water. The stained smear was air dried at room temperature and observed under oil immersion field (100x objective) of Leica light microscope. One thousand sperms were examined, and they were categorized into five broad categories; Normal, head abnormalities, neck abnormalities, tail abnormalities and multiple anomalies. The sperms were identified as normal if the sperm under analysis showed no head, neck, tail or multiple abnormalities mentioned below. Sperms under head abnormalities category were subdivided into Headless – if the sperm appeared only as tail without the head, flattened head – the sperms which exhibited varying degrees of curvature of the head and sometimes failing to exhibit any curvature. Pinhead – the head of the sperms which resembled the head of a pin or a nail and some were offset or slanting. The bent neck was the subdivision used to categorize the sperms with neck abnormalities. The sperms appeared to exhibit varying degrees of bending ranging from slight to greater than 180° were considered to have a bent neck. The bent tail was the only subdivision used to categorize the sperms with tail abnormalities. The sperms which exhibited eccentric insertions at the junction of midpiece or tail and seen as bending or coiling at the tip of the tail were considered to have a bent tail. The sperms which exhibited a combination of mentioned above head, neck and tail abnormalities were grouped under multiple abnormalities category. The results obtained were expressed in percentages. Free heads were excluded from the analysis as they are usually seen in sperm smears [13,14].
Testicular and Epididymal Histology: The excised left testes and epididymis were fixed in Bouin’s solution and processed using an automated tissue processor. The tissue was embedded in paraffin blocks, sectioned perpendicularly in longitudinal axis with 5 μm thickness, and stained with Haematoxylin and Eosin (H&E) staining technique. The stained slides were mounted using DPX mounting media and were evaluated for abnormal histology.
Statistical Analysis
Quantitative data (body weight, sperm count etc.,) were compared among the low and high exposure groups against the control group by the use of levene’s test of homogeneity of variances. If levene’s test indicate lack of homogeneity (p<0.001) Welch ANOVA was used otherwise ANOVA was applied to selected measures from this study.
Jonckhere Terpstra test for linear trend was used to determine the significance of the dose-response relationship. When a significant (p<0.05) main effect for dosage occurred, Tukey HSD pairwise comparison test was used. The data were tabulated and analysed using SPSS version 15.0 for Windows.
Results
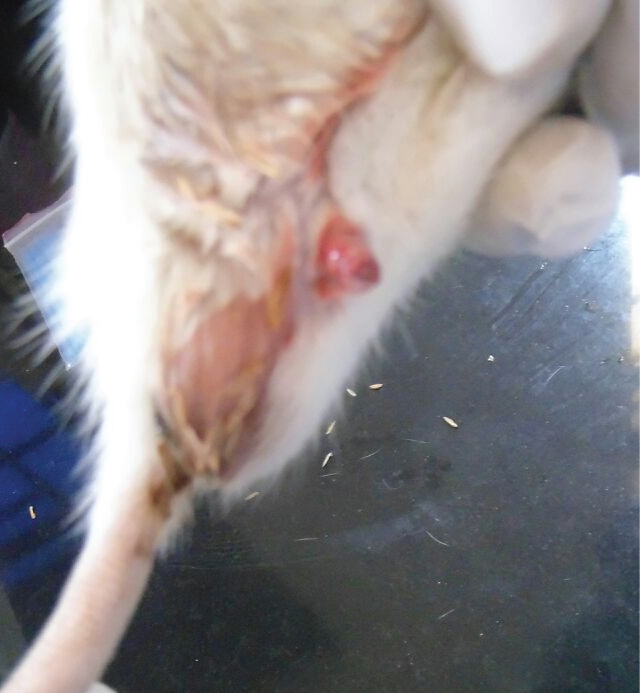
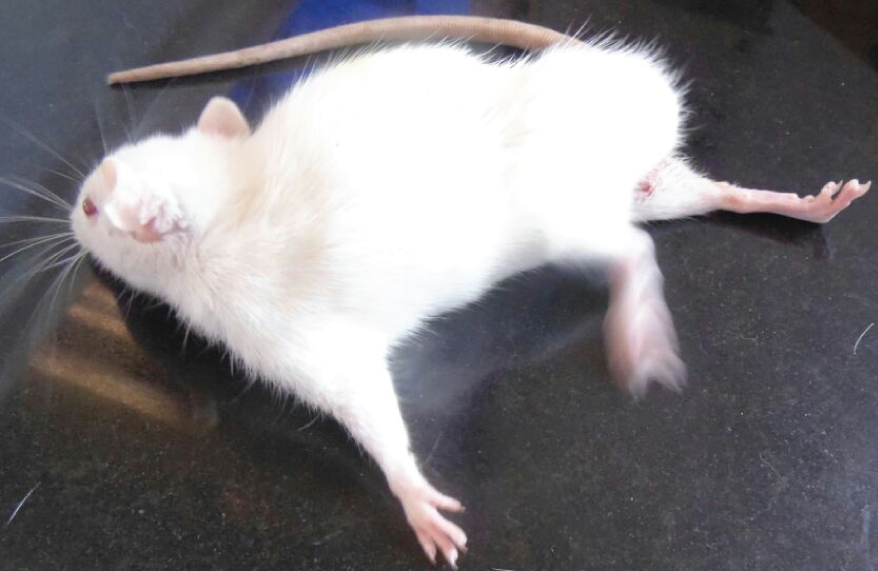
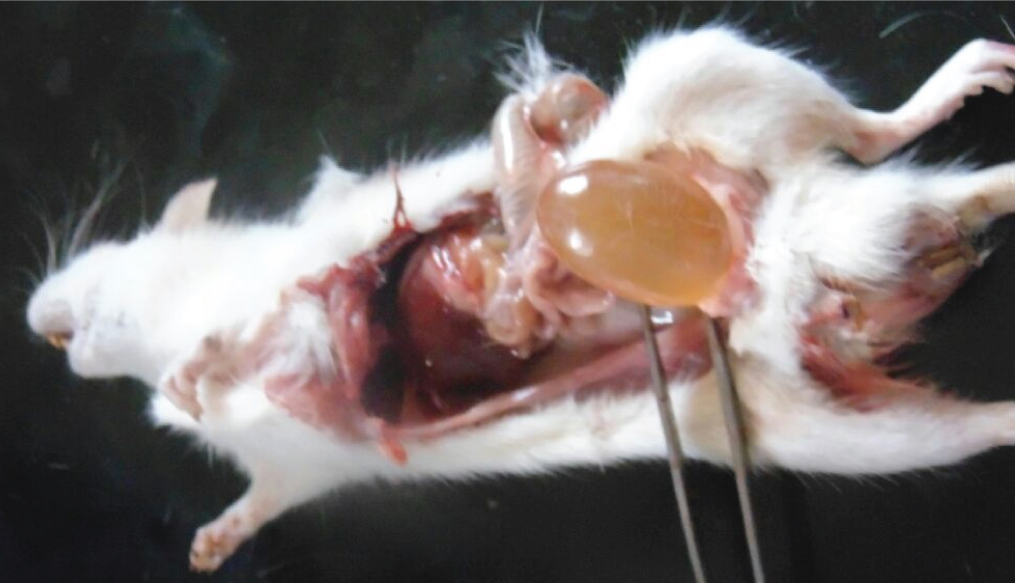
The high dose acrylamide treated rats showed alterations such as decreased urine excretion, anal lesions [Table/Fig-1], hind limb splaying [Table/Fig-2], and urinary bladder enlargement after sacrifice [Table/Fig-3]. The ingestion of acrylamide for 21 days in animals didn’t show any significant body weight change till day 11. On the day 11, there was a observable change in the body weight in acrylamide treated groups compared to control [Table/Fig-4]. After the 17th day of exposure, two animals in high dose group were observed dead. Hence, the body weight decreases on day 17 (p=0.002) which was significantly important. The high dose animals showed visible anatomical lesions in testis hence the treatment was terminated on day 21 and the analysis was carried out for average of four animals for high dose groups. The significant decrease in body weight (p<0.001) was observed on day 21 with dose dependent manner.
High dose group animal showed anal lesions.
High dose group animal showed hind limb splaying.
High dose group animal showed decreased urinary excretion on day 17 to day 20 and urinary bladder enlargement was observed after sacrifice.
Impact of low and high dose acrylamide on body weight gain compared with control using one-way ANOVA (Data expressed in Mean±SEM, p<0.05, is considered as significant, *indicates significant difference with control, ‘$’ indicates significant difference with the low dose).
| Body weight (in g) |
|---|
| Control | Low dose | High dose |
|---|
| day_0 | 170±17.321 | 168.25±3.266 | 166.43±7.883 |
| day_11 | 211±14.434 | 196.38±3.412 | 180±5.598 |
| day_17 | 231.67±11.260 | 207.75±3.534 | 185.71±6.931*$ |
| day_21 | 241±10.970 | 216.63±3.770 | 168.40±12.416*$ |
The wet organ weight of right epididymis (p=0.001) and left epididymis (p=0.002) were significant in high dose group as compared to control and low dose [Table/Fig-5]. The significant decrease in relative wet organ weight were observed in animals treated with high dose acrylamide. A linear dose-response relationship was observed in the left and the right epididymis (p=0.006 and p=0.002). However, there was no significant difference in the wet weights of testis.
Impact of low and high dose acrylamide on relative wet organ weight compared with control using one-way ANOVA (Data expressed in Mean±SEM, p<0.05, is considered as significant, *indicates significant difference with control, ‘$’ indicates significant difference with the low dose).
| Relative wet organ weight (in g) |
|---|
| Control | Low dose | High dose |
|---|
| Right Testis | 1.287±0.062 | 1.217±0.044 | 1.127±0.045 |
| Left Testis | 1.300±0.009 | 1.244±0.048 | 1.115±0.036 |
| Right Epididymis | 0.454±0.283 | 0.432±0.137 | 0.282±0.369*$ |
| Left Epididymis | 0.447±0.034 | 0.378±0.016 | 0.280±0.025*$ |
The toxicological effects of acrylamide on male rats reproductive functions were assessed by the sperm reserves in cauda epididymis, sperm motility, and sperm morphology [Table/Fig-6]. A linear dose-response relationship in sperm count was observed (p<0.001). In pair wise comparison, a significant decrease in the sperm count among the high dose as compared to the low dose and the control groups (p<0.001 each) was observed. From the epididymis, sperm motility test found prominent decreased percentage of motile sperms in high dose group (1%) compared to low dose (20%) and control groups (60%). As other quantitative measures, a significant difference in sperm morphological change (p<0.001) is observed in high dose as compared to low dose and controls. The rats in the high dose group showed morphological defects including headlessness, absence of tail, flattened head, pin head, bent neck and bent tail.
Impact of low and high dose acrylamide on Sperm count, Sperm non-motility percentage and Sperm morphology percentage compared with control group using one-way ANOVA (Data expressed in Mean±SEM, p<0.05, is considered as significant, *indicates significant difference with control, ‘$’ indicates significant difference with the low dose).
| Parameters | Control | Low dose | High dose |
|---|
| Sperm Count | 8436666.67±687764.091 | 7936875.00±349678.344 | 4026000.00±481698.567*$ |
| Non-Motile sperm (%) | 42.00±6.110 | 80.25±3.310* | 99.20±0.374*$ |
| % of Normal Sperm Morphology | 93.17±1.364 | 91.50±0.700 | 81.70±0.578*$ |
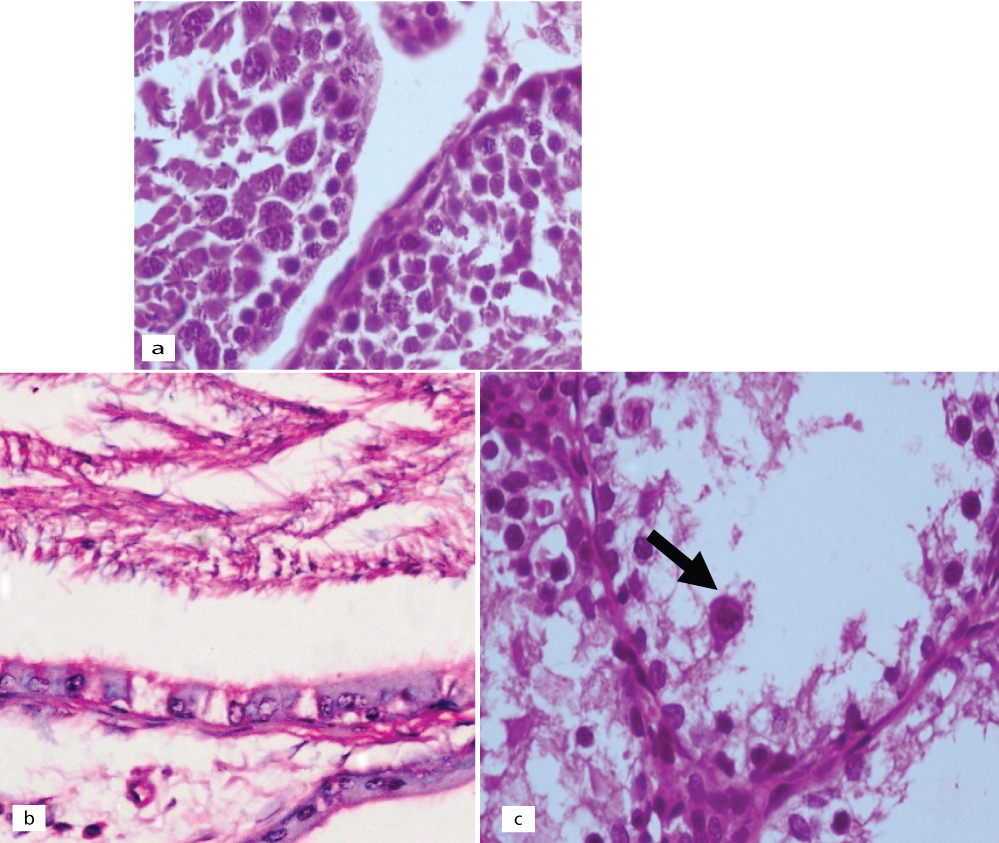
Control animal testis showed the normal testicular biopsy, epididymis with normal morphology and seminiferous tubule with normal germ cell maturation [Table/Fig-7]. It was noted that the rats exposed to a low dose of acrylamide [Table/Fig-8], had mild to no effects on the size and number of the seminiferous tubules as compared to the controls. Few showed a maturation arrest at the level of spermatogonia to spermatids. No sloughing of the germ cells into the lumen was seen. The effect on the epididymis ranged from reduced luminal sperm capacitance volume to a vacuolar degeneration of lining epithelium. In contrast to the controls, the rats exposed to a high dose [Table/Fig-9], showed mild to moderate atrophy of the seminiferous tubules with a modest to an occasional one showing reduced luminal diameter. The salient effects on the gonocytes included a maturation arrest affecting the spermatogonia and the spermatids [Table/Fig-9a]; but more visibly, causing sloughing and apoptosis of the germ cells [Table/Fig-9b,c]. The effects on epididymis included a reduced sperm capacitance volume and vacuolar degeneration of the basal epithelium.
Haematoxylin & Eosin of control animals: (a) seminiferous tubules showing regular diameter of seminiferous tubules with normal sequence of gametogenesis (H&E; x100); (b) cross-section of the of epididymis showing normal morphology with pseudostratified columnar lining with stereocilia and luminal spermatozoa (H&E; x100); (c) cross-section of seminiferous tubule at the level of mid-testis, cut longitudinally and shows normal maturation sequence of spermatogenesis: spermatogonia, spermatids and luminal spermatozoa (H&E; x100).

Haematoxylin & Eosin of low dose treated animals: (a) epididymis showing reduced luminal capacitance and few columnar cells with cytoplasmic vacuoles (H&E; x400); (b) testis showing focal maturation arrest of spermatogenesis (H&E; x200).
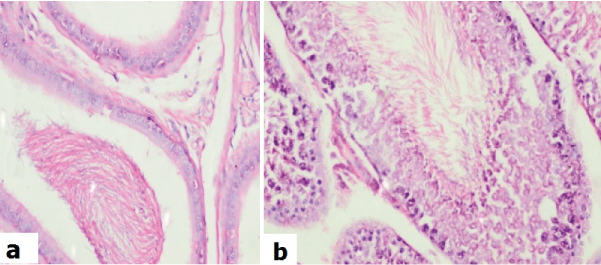
Haematoxylin & Eosin of high dose treated animals: (a) testis showing sloughing of germ cells into the lumina (H&E; x400); (b) epididymis showing lining columnar cells with cytoplasmic vacuoles (H&E; x400); (c) testis showing prominent apoptosis (arrow) and sloughing of germ cells into the lumina (H&E; x400).
Discussion
In the present study, the effect of acrylamide toxicity on the reproductive organs of adult male Wistar rats were evaluated. Body weight is a vital non-specific indicator systematically reflecting the toxicity of substances [15], and can be used to assess the effect of toxicity on the growth status of rats. This study reports the significant decrease in body weight in high dose group from the day 17 to day 21 and the decrease is dose dependent. The two animals found dead on day 18 which could be another detrimental effect of high dose acrylamide on rats. The remaining animals in high dose showed decreased urinary output on day 19 to day 20 with anal lesions and hind limb splaying. Hence the exposure of high dose acrylamide could lead to life threatening effect, which could involve multi-organ dysfunctions.
Several experimental studies indicated the detrimental impact of acrylamide on male fertility indices, including sperm parameters and histopathological examination with different doses and days of exposure. Tyl RW et al., showed significant reduction in weight gain, and non-significant effects on sperm motility or sperm count at 5mg doses [16], and another study showed significant reduction in epididymal weight in acrylamide-treated groups and a significant decrease in number of sperms in cauda epididymis, which was dose-dependent with the doses above 40 mg/Kg body weight. They also observed a reduction in testicular weight in 60 mg/Kg body weight [17]. In this study, similar findings including the significant decrease in body weight, right epididymis and left epididymis relative wet organ weights in both low and high dose acrylamide exposures was observed. The decrease in relative wet weights of right and left testis was non-significant but observable decrease in dose dependent manner. Concerning sperm count, the current result is also in agreement with the study of Wang H et al., who showed that acrylamide could decrease the concentration of epididymal sperm concentration [10]. Sperm count showed significant decrease in both low and high dose treated groups. A previous report says that the chronic exposure to acrylamide with doses 10 and 18 mg/kg can affect sperm development and increased defects in sperm morphology [18], but the present study observed significant alterations in the sperm development, sperm motility and morphology in low and high dose acrylamide exposure. The reason for this effect can be due to the transformation of acrylamide to glycidamide, which is a major step in acrylamide-induced mutation in germ and somatic cells [19,20]. Studies showed that acrylamide produces sperm head abnormalities in rodents [21,22]. We also observed a similar trend among low and high dose with increased head abnormalities. Tyl RW et al., studied the effect of acrylamide on male animals and observed reduced sperm count, abnormal sperm morphology, and degeneration of the epithelial cells of the seminiferous tubules in testis, and as a result decreased rates of fertility [8].
Histological reports are in agreement with the above findings, showing testicular maturation arrest and apoptosis of gonocytes which may also contribute to decreased fertility in high dose groups. The acrylamide toxicity may be attributed to the effect on the kinesin motor proteins that occurs in the flagella of sperm causing reduced sperm motility [8,17]. The high dose animal group showed 100% non-motile sperms; this may be due to the toxic effect of acrylamide on flagellar kinesin motor proteins. Our findings in the histopathological evaluation of testis were in agreement with the previous studies that showed changes such as swelling, vacuolations, necrosis of the spermatids, increased apoptotic cells, and formation of multinucleated giant cells in the seminiferous tubules after acrylamide consumption [18,23]. The histopathological evaluation of low dose acrylamide on epididymis showed reduced luminal capacitance and few columnar cells with cytoplasmic vacuoles and testis revealed focal maturation arrest of spermatogenesis. The high dose groups showed epididymis showing lining columnar cells with cytoplasmic vacuoles, testis showing prominent apoptosis and sloughing of germ cells into the lumina. The present study findings suggests that even low dose acrylamide contributes to observable and significant reproductive toxicity which directs the human attention to reduce the consumption of starchy food and other carbohydrates, cooked at high temperature.
Limitation
The present study could not include the intermediate dose levels of acrylamide in this study that would give a clearer picture about the level of toxicity. Though the histopathological evaluation could confirm the male reproductive toxicity of acrylamide, further molecular studies are needed to understand the mechanism behind this toxic effect.
Conclusion
The present study showed that acrylamide exposure results in altered testicular and epididymal architecture and sperm parameters which may lead to significant functional reproductive disorder. The lethality of acrylamide was in a dose-dependent manner, causing the reproductive toxicity by affecting the sperm volume, maturation, and leading to apoptosis of gonocytes. These abnormalities were in pact to the histopathological changes.