Epithelioid Haemangioendothelioma (EHE) is an extremely rare vascular tumour that develops in the soft tissues or in viscera such as liver, lung, bone, brain, spleen or even the small intestine. The tumour has an intermediate malignant potential. Hepatic Epithelioid Haemangioendothelioma (HEHE) is often not correctly diagnosed and may be confused with benign hepatic cavernous haemangioma or even with hepatic metastatic deposits. Imaging plays an important role in accurate diagnosis of this unusual entity with Dynamic contrast enhanced CT and MR showing the characteristic diagnostic features of HEHE and assist in differentiating it from other hepatic masses such as cavernous haemangioma, cholangiocarcinoma and metastasis. The differentiation is important for appropriate therapeutic triage as once detected these mesenchymal masses cannot be ignored, albeit need to be managed aggressively. Treatment modalities for this mesenchymal tumour include hepatic resection, orthotropic liver transplantation (even in cases with known metastases), radiotherapy and chemotherapy with Adriamycin, Doxorubicin or Interferon. Present case had an innocuous and beguiling clinical presentation and hepatic, pulmonary as well as unilateral adrenal masses were detected on dynamic CT and MR imaging. Metastases and cavernous haemangioma were ruled out based on the imaging appearance and advised vascular tumour markers to confirm the diagnosis of multifocal HEHE. After this, CD34 and CD31 markers were found positive, patient was referred for hepatic resection. A biopsy was conducted, that demonstrated cords of endothelial and spindle cells with large sinusoids, typical of EHE. Patient subsequently underwent a trisegmental right lobe resection (since right hepatic lesions were more numerous), followed by chemotherapy as well as radiotherapy.
Black target sign, Diffusion magnetic resonance, Dynamic contrast enhanced computed tomography, White target sign
Case Report
A 12-year-old boy presented to the Emergency department in our hospital with moderate dull aching right upper quadrant pain since seven months. The pain was aggravated on effort and subsided with NSAIDs. On physical examination, there was mild non-tender hepatomegaly. Laboratory studies revealed normal blood counts and liver enzymes were in the normal range. As part of the initial work-up, an ultrasound abdomen was performed. This revealed few well-defined round hypoechoic hepatic masses in a sub-capsular location. No obvious vascularity was noted within these lesions on Doppler colour flow imaging.
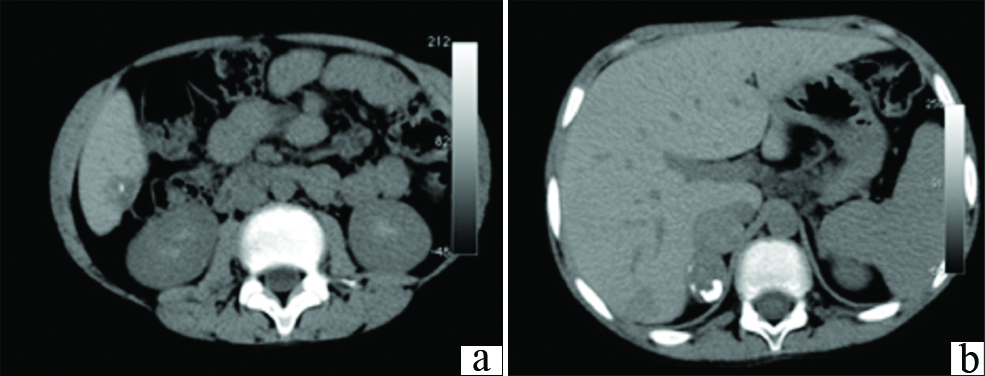
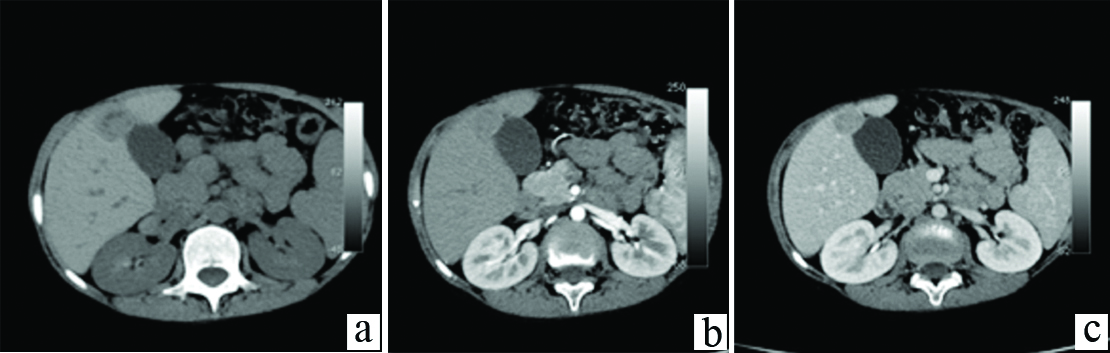
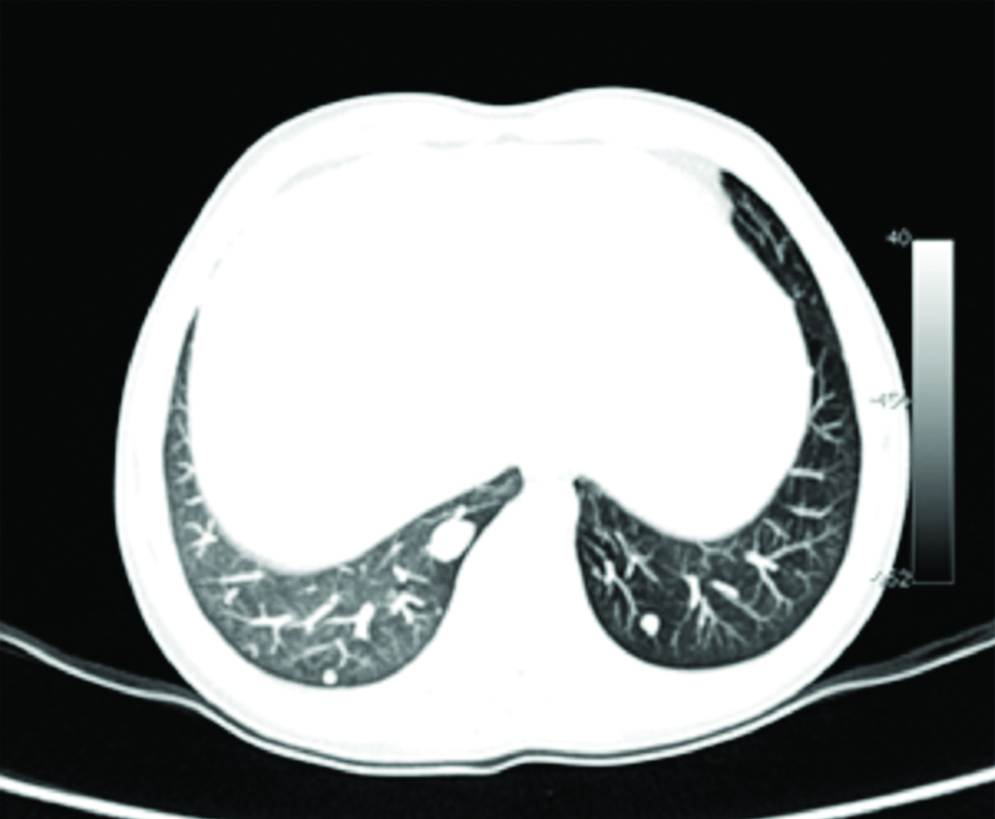
For a more comprehensive evaluation of the liver, CT was performed. Unenhanced CT abdomen revealed few well defined predominantly sub capsular round hypo dense hepatic masses of sizes 2-4 cm; and similar nodules in the right adrenal gland, with punctate calcific foci within the adrenal lesions [Table/Fig-1a,b]. A multiphase dynamic CECT was performed. On arterial phase images of the dynamic study acquired at 20 seconds post bolus injection, the hepatic and adrenal lesions showed faint peripheral enhancement. The portal phase acquired at 60 seconds and delayed phases of the dynamic study at 180 to 220 seconds demonstrated progressing significant enhancement in the central part of the lesions, with the peripheral enhancement becoming more prominent [Table/Fig-2a-c]. The CECT examination was extended to include the chest, and revealed multiple enhancing pulmonary nodules in a sub pleural distribution, predominantly involving bilateral lower lung lobes [Table/Fig-3].
a) Subcapsular hypo attenuating nodular lesion with calcific foci within in segment VI of liver; b) Multiple nodular hypodense lesions in the right adrenal gland with calcific foci.
a) Unenhanced image reveals a hypodense (HU-54) lesion in segment V of liver; b) Arterial phase imaging reveals subtle target like enhancement more in the centre than in the periphery; c) Delayed phase image reveals progressive enhancement of the lesion.
Lung window CT image reveals few subpleurally distributed peribronchovascular nodules in bilateral lung fields suggestive of metastases.
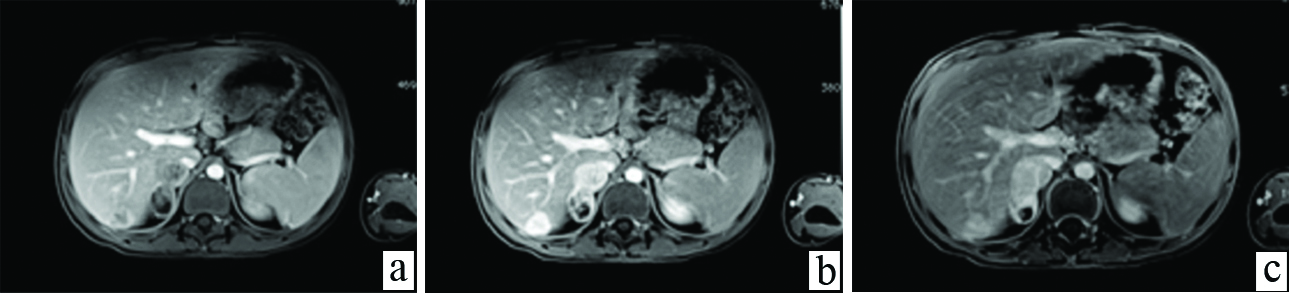
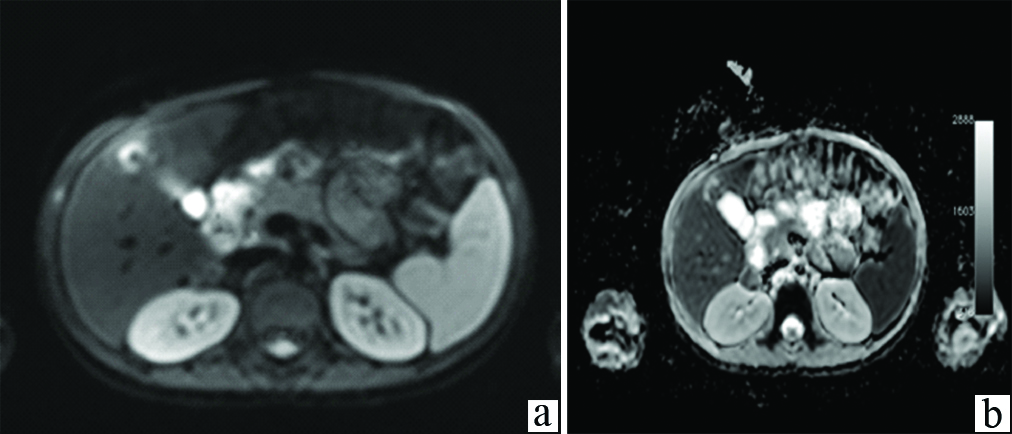
Abdomen MR imaging was conducted for better characterization of the hepatic and adrenal lesions and to conclusively differentiate them from haemangiomas or metastases. On MR imaging, these liver lesions appeared hypointense on T1-weighted images and isointense on T2 weighted images to hepatic parenchyma. Two large well defined lesions with similar signal intensity as the hepatic lesions were seen in the right adrenal gland. Dynamic contrast-enhanced MR imaging was performed. On arterial phase of dynamic MR acquired at 30 seconds after IV bolus administration, a complete thick rim of peripheral enhancement was detected in both, hepatic and adrenal nodules. Portal phase demonstrated persistent peripheral enhancement with marked enhancement in the central part of these nodular lesion. On delayed MR images at five minutes, and 10 minutes, enhancement was seen to persist in the central and gradually reduce in the peripheral part of the lesions [Table/Fig-4a-c]. DWMR demonstrated restricted diffusion in the central part of lesions, appearing hyper intense, with hypointense signal in the corresponding ADC images [Table/Fig-5a,b]. These imaging features of multiple nodules within the liver and unilateral adrenal gland, which demonstrated early arterial phase enhancement on dynamic CT and MR in the lesion periphery, progressing to center of lesion in portal phase, with delayed images showing dense persistent enhancement central as well as peripheral, without any washout; along with multiple nodules in bilateral lower lung fields and right adrenal gland, lead to an imaging diagnosis of multifocal EHE with concomitant hepatic, pulmonary and right adrenal involvement. Serum levels of vascular tumour markers CD34 and CD31 were found to be positive. The adrenal and pulmonary lesions appeared to be due to metastatic spread of HEHE. Diffuse hepatic involvement in the form of multiple nodules in both lobes, that have a tendency to coalesce, has been described as diagnostic of HEHE in literature. Metastases were excluded since these lesions showed persistent enhancement in the central and peripheral part unlike metastasis which show early washout and appear hypodense/hypointense on portal phase scans. Cavernous haemangioma was ruled out due to multifocal involvement and presence of pulmonary as well as adrenal lesions. The patient was referred for hepatic resection after a liver biopsy proved the diagnosis of HEHE.
a) Late arterial phase dynamic contrast enhanced T1-weighted fat suppressed images showing peripheral ring like enhancement in the hepatic and adrenal lesions -“the black target sign”; b) In portal phase lesions show progressively increasing enhancement in the central part of lesions; c) 3 minute delayed dynamic MR image shows decreasing peripheral and increasing central enhancement in the lesions.
Diffusion weighted MRI image show restricted diffusion in part of the lesion. a) DW Image; b) ADC image.
Discussion
Epithelioid Haemangioendothelioma (EHE) is an uncommon tumour of vascular origin first described in 1982 by Weiss and Enzinger [1] The recent World Health Organization classification describes EHE as lesions that fall into the category of locally aggressive tumours with metastatic potential [2]. The histopathological appearance is of dendritic and epithelioid-appearing endothelial cells in a fibrous stroma. Hepatic EHE can be seen as a solitary nodule, multiple nodules or the diffuse form with multiple coalescing nodules that tend to be more prominent in the hepatic periphery. HEHE is a solid tumour with intermediate malignant potential; the prognosis is not as bad as that of malignant vascular angiosarcoma, nor as indolent as that of the benign cavernous haemangioma. This tumour is extremely rare with an incidence of less than 0.1 per 100,000 and is found in the adult population with a female predominance and male female ratio of 2:3 [3]. An aetiological correlation with vinyl chloride, asbestos, and thorotrast exposure has been described [4]. A hypothesis suggests and refers to a causal relationship between chronic Bartonella infection and the pathogenesis of this rare vascular tumour. However, more recently chromosomal translocation involving chromosomes 1 and 3 has been detected to be the causative factor [5]. The clinical presentation tends to be vague and nonspecific with right upper quadrant pain and weight loss. Liver enzymes, serum bilirubin, alkaline phosphatase and aspartyl aminotransferase may be normal or elevated. Although routine epithelial tumour markers such as CEA 19 are negative, specific vascular endothelial tumour markers namely CD 34 and CD 31 are often positive [5]. Apart from liver, EHE have been described in other viscera such as lungs, spleen and peritoneum. Mortality is 13% when EHE is located in soft tissues, 35% when it affects the liver, and 65% if it reaches the lung [5].
The diagnosis is essentially based on imaging. Imaging techniques, sonography, and contrast enhanced CT or MR are very useful to diagnose these tumours. Lesions of hepatic EHE may be solitary or multifocal and diffuse, with the solitary form being less common. Typically the lesions are located sub marginally along the liver periphery [6]. In the nodular pattern single or multiple discrete peripheral sub capsular hepatic nodules are found, while in the diffuse form the numerous peripherally located nodular lesions tend to coalesce to form a mass that appears to be infiltrating. Capsular retraction and lobulation of the hepatic surface are typical findings seen in the diffuse pattern, described as the “capsular retraction” sign, caused by a fibro proliferative reaction induced by the tumour, which leads to invagination of the adjacent liver capsule [6]. Calcification is considered a common feature with an incidence of 15-25%. On ultrasound, lesions of HEHE are seen to be discrete or confluent hypo echoic masses and can mimic metastases. This appearance is distinctively different from that of cavernous haemangiomas which are always hyper-echoic on USG.
On NCCT abdomen, the nodules appear hypo dense to the hepatic parenchyma, and demonstrate peripheral enhancement in the arterial phase, with characteristic stronger homogenous enhancement within the lesion center in the portal and hepatic phases. Multiphase dynamic CECT is preferred since on routine CECT the correct diagnosis may not be possible since acquisition is mostly in the portal phase alone. “Target-like” enhancement, denser in the central part of nodule has been described in the portal phase of dynamic CT, due to contrast entrapment within the fibrous core [7].
On T1W MR, HEHE lesions exhibit low signal intensity, and on T2-weighted images appear heterogeneously hyper intense relative to the normal liver parenchyma. The size of nodules varies from 5 mm to 10 cm with capsular retraction more often seen when nodules are >2 cm. Dynamic contrast-enhanced MR can be very useful in accurate diagnosis of HEHE and superior to CECT for detecting smaller sub marginal lesions. The enhancement patterns may differ with the lesion size-nodules smaller than 2 cm demonstrate homogenous arterial phase enhancement without progression, nodules 2-5 cm in size demonstrate peripheral ring like arterial phase enhancement along with progressive and persistent enhancement in delayed phase; while those larger than 3 cm typically demonstrate progressive heterogeneous enhancement best appreciated on delayed scans [8]. Recently on arterial phase dynamic MR, the ‘black target sign’ has been described as characteristic of HEHE, where a rim of peripheral enhancement with lesser enhancement in the central part of lesion resulting in central low signal intensity is seen; while ‘the white target sign’ has been described in the portal venous and delayed dynamic MR phases, when central part of the lesion demonstrates homogenous enhancement with contrast washout from the periphery [9].
Apart from these patterns, HEHE may demonstrate a ‘strip’ of parenchymal enhancement coursing along the hepatic vessels in the arterial phase, which becomes more prominent in the portal and delayed phases. Sometimes tapering of portal vein and hepatic vein branches towards the enhancing tumour nodules can be detected on CEMR, which is described as the ‘lollipop sign’ and is also characteristic of HEHE [8,9]. These patterns are not seen in metastases or cavernous haemangioma on dynamic MR. Due to endothelial cellular proliferation in these tumours, restricted diffusion within the central part of lesion is described on DWMR, seen as hyper intensity and corresponding hypointense signal on the ADC diffusion maps, and differentiates the nodules of HEHE from metastatic nodules [10].
Extra hepatic EHE may be intraabdominal, intrathoracic or intraosseous. The most characteristic presentation of thoracic EHE is presence of bilateral multiple nodular pulmonary opacities. Radiologically, pulmonary EHE appearing as multiple perivascular nodules ranging in size up to 2 cm in diameter (most are less than 1 cm), with well-defined or blurred margins predominantly located in the lower lobes [11]. These show little or no growth on serial chest radiographs or computed tomography. Intraabdominal EHE manifests with peritoneal, omental or lymph node involvement [6]. Adrenal involvement in the form of stromal tumours is extremely rare and has not been described to the best of our knowledge.
Since HEHE demonstrates minimal peripheral enhancement in the arterial phase of dynamic CT or MR; with progressive centripetal lesion enhancement in the portal and delayed phases, similar to cavernous haemangioma, it is important to differentiate between the two. This differentiation is possible if the radiologist remembers that sonographic appearance of cavernous haemangiomas is typically echogenic, thus different from the hypoechoic nodules of HEHE. Moreover, haemangiomas are iso dense to hepatic parenchyma on CT and iso intense on T2W MR and are not associated with pulmonary, abdominal visceral or peritoneal lesions. Hepatic metastasis and cholangiocarcinoma are included in the differential diagnosis of HEHE. Metastasis show typical arterial phase enhancement, with a washout in portal phase of dynamic CT and MR, without enhancement in the delayed phase [12]. Cholangiocarcinoma is clinically associated with raised CEA 19 levels and on imaging dilatation of biliary radicles is always associated with delayed phase enhancement and capsular retraction (since it grows along the bile ducts) [3,12]. Adrenal involvement does not occur unless adjacent organ invasion is seen in advanced stages.
The surgical management of HEHE tumours is in the form of hepatic resection, or hepatic transplantation with one year and five year survival rates of 54.5% and 96% respectively. Liver transplantation is recommended as the treatment of choice due to multicentricity of HEHE [3,9,13]. Chemotherapy agents employed have been doxorubicin, vincristine, interferon-alpha, 5-FU, and thalidomide. A newer chemotherapy alternative includes Vascular Endothelial Growth Factor (VEGF) targeted therapy. Survival rates with chemotherapy and radiotherapy have been reported to be 73% and 30% respectively.
Conclusion
Hepatic EHE is an unusual vascular tumour which commonly presents as multiple hepatic nodules. Extra hepatic involvement is also common and represents dissemination. In view of overlapping imaging features with cavernous haemangioma, metastases and cholangiocarcinoma, it is essential to establish the diagnosis conclusively with the help of the dynamic CECT/CEMR imaging and sonography. Disseminated multifocal EHE must be considered when hepatic as well as extra hepatic nodular masses showing arterial as well as delayed phase enhancement are encountered especially in the absence of a known primary malignancy. Present case was HEHE with dissemination to lungs and the adrenal gland.
[1]. Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW, Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 casesHum Pathol 1984 15:839-52.10.1016/S0046-8177(84)80145-8 [Google Scholar] [CrossRef]
[2]. Mertens F, Unni K, Fletcher CDM, World Health Organization classification of tumoursPathology and genetics. Tumours of soft tissue and bone 2002 LyonIRAC Press:155 [Google Scholar]
[3]. Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapyCancer 2006 107(9):2108-21.10.1002/cncr.2222517019735 [Google Scholar] [CrossRef] [PubMed]
[4]. Agrawal N, Parajuli S, Zhao P, Satoskar R, Laurin J, Azumi N, Liver transplantation in the management of hepatic epithelioid hemangioendothelioma: a single-center experience and review of the literatureTransplantation Proceedings 2011 43(7):2647-50.10.1016/j.transproceed.2011.06.03521911139 [Google Scholar] [CrossRef] [PubMed]
[5]. Mendlick MR, Nelson M, Pickering D, Johansson SL, Seemayer TA, Neff JR, Translocation t (1; 3) (p36.3; q25) is a nonrandom aberration in epithelioid hemangioendotheliomaAm J Surg Pathol 2001 25(5):684-87.10.1097/00000478-200105000-0001911342784 [Google Scholar] [CrossRef] [PubMed]
[6]. Lyburn ID, Torregiani IC, Harris AC, Zwirewich CV, Buckley AR, Hepatic endotheloid hemangioendothelioma sonographic, CT and MR imaging appearancesAJR 2003 180(5):1359-64.10.2214/ajr.180.5.180135912704052 [Google Scholar] [CrossRef] [PubMed]
[7]. Kim EH, Rha SE, Lee YJ, Yoo IR, Jung ES, Byun JY, CT and MR imaging findings of hepatic epithelioid hemangioendotheliomas: Emphasis on single nodular typeAbdom Imaging 2015 40:500-09.10.1007/s00261-014-0229-325179888 [Google Scholar] [CrossRef] [PubMed]
[8]. Zhou L, Cui MY, Xiong J, Dong Z, Luo Y, Xiao H, Spectrum of appearances on CT and MRI of hepatic epithelioid hemangioendotheliomaBMC Gastroenterology 2015 15:6910.1186/s12876-015-0299-x26088585 [Google Scholar] [CrossRef] [PubMed]
[9]. Gan LU, Chang R, Jin H, Yang Li, Typical CT and MRI signs of hepatic epithelioid hemangioendotheliomaOncol Lett 2016 11(3):1699-706.10.3892/ol.2016.414926998064 [Google Scholar] [CrossRef] [PubMed]
[10]. Ruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ, Hepatic epithelioid hemangioendothelioma: Findings at CT and MRI including preliminary observations at diffusion-weighted echo-planar imagingAbdom Imaging 2011 36:415-24.10.1007/s00261-010-9641-520730424 [Google Scholar] [CrossRef] [PubMed]
[11]. Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M, Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumourOncol Rev 2014 8(2):25910.4081/oncol.2014.25925992243 [Google Scholar] [CrossRef] [PubMed]
[12]. Albarello F, Di Stefano F, Vennarecci G, Ettorre G, Rizzi EB, Campioni P, Diagnostic imaging of the diffuse hepatic epithelioid hemangioendothelioma’s type: a case reportJ Clin Case Rep 2016 6:84910.4172/2165-7920.1000849 [Google Scholar] [CrossRef]
[13]. Mistry AM, Gorden DL, Busler JF, Coogan AC, Kelly BS, Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendotheliomaJ Gastrointest Cancer 2012 43:521-25.10.1007/s12029-012-9389-y22544493 [Google Scholar] [CrossRef] [PubMed]