Hypocellular Acute Myeloid Leukaemia- A Diagnostic Conundrum
Sindhura Lakshmi Koulmane Laxminarayana1, Chethan Manohar2
1 Assistant Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Udupi, Karnataka, India.
2 Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Udupi, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sindhura Lakshmi Koulmane Laxminarayana, Assistant Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Udupi-576104, Karnataka, India.
E-mail: sindhura.lakshmi@manipal.edu
Hypocellular acute leukaemia is a rare entity with an incidence of 5-12% of all acute leukaemias. Almost all reported cases are of myeloid lineage. It is defined by bone marrow hypocellularity (less than 20% of cellularity in trephine biopsy) with increase of bone marrow blasts (20% or more) and peripheral blood blastopenia. It is termed as a secondary disease in a pre-existing haematologic disorder or prior cytotoxic or radiation therapy and is often misdiagnosed as Myelodysplastic Syndrome (MDS). In this report, we describe an unusual case of Hypocellular Acute Myeloid Leukaemia (H-AML) without any past haematologic disease or radio/chemotherapy in an elderly male. The diagnosis of H-AML can be difficult and may be confused with Hypocellular MDS (H-MDS) and Aplastic Anaemia (AA). Presence of dysplasia and abnormal localization of immature precursors may not be able to differentiate between H-AML from H-MDS. Strict diagnostic criteria must be followed to arrive at correct diagnosis which includes counting atleast 100 leukocytes on peripheral smear and 500 leukocytes in Bone Marrow Aspirate (BMA).
Bone marrow aspirate, Flow cytometry, Hypocellullar-myelodysplastic syndrome
Case Report
A 62-year-old previously healthy male who developed progressively worsening generalized fatigue, after blood and bone marrow examination at an outside hospital, he was diagnosed to have MDS and was referred to Kasturba Hospital, Manipal for treatment. On examination, there was no hepatosplenomegaly, bleeding or severe pallor. Review of systems was normal.
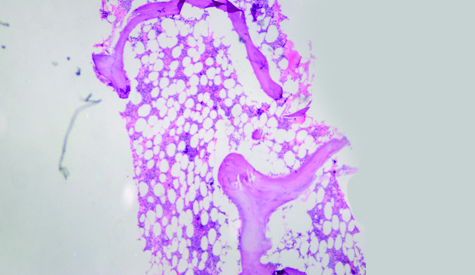
Haemoglobin was 11.6 g/dL; platelet count: 374.0x103/μL; total WBC: 6.5x103/μL. Peripheral smear showed 11% blasts which were emarginated at the tail end of the smear [Table/Fig-1]. BMA had hypocellular (<20% cellularity) marrow particles with markedly suppressed normal haematopoiesis [Table/Fig-2]. All nucleated cell differential count revealed 54% blasts; 7% promyelocytes; 4% myelocytes; 5% metamyelocytes and 13% neutrophils with dyspoiesis in the granulocyte series. Cytochemistry for Myeloperoxidase (MPO) and Sudan black B was positive in blasts [Table/Fig-3a,b]. Periodic acid Schiff stain showed diffuse positivity. Hence, the case was categorized as H-AML without maturation. Bone marrow biopsy revealed hypocellular marrow (<20% cellularity) [Table/Fig-4] with interstitial infiltration of blasts. Erythropoiesis and megakaryopoiesis were suppressed. No evidence of dysplasia was noted.
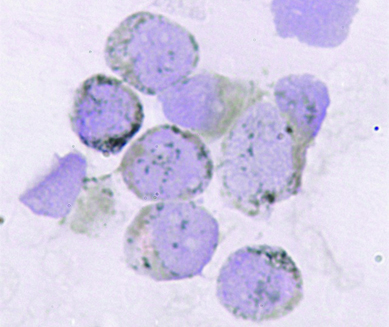
Peripheral smear, Leishman, X400. Myeloblasts with intermediate nucleo-cytoplasmic ratio, indented nuclei and coarse chromatin, 2-3 nucleoli, vacuolated cytoplasm are seen.
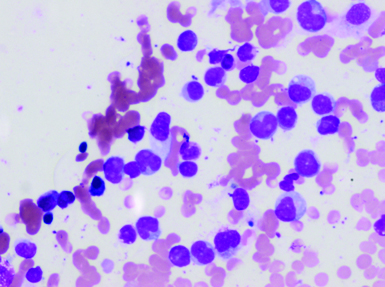
Bone marrow aspirate, Leishman, X100. <20% marrow cellularity with diluted cell trails.
Bone marrow aspirate, myeloperoxidase, X100. Positive in blasts.
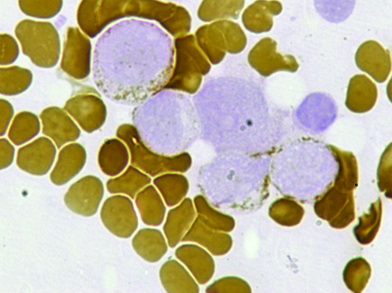
Bone marrow aspirate, Sudan black B, X100, Positive in blasts.
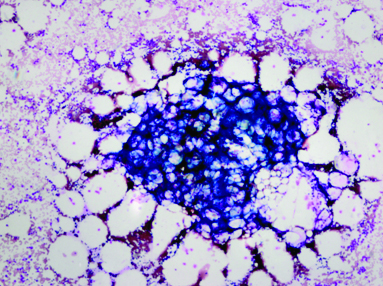
Bone marrow biopsy, haematoxylin and eosin, X40. <20% marrow cellularity and thin trabecular bone.
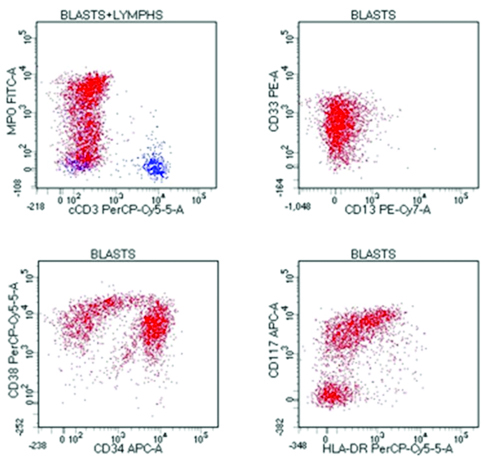
Flow cytometry evaluation was performed on BMA. All reagents, flow cytometer and software were acquired by Becton Dickinson (BD) biosciences, USA. Sample tubes were prepared by bulk dilution method so that atleast one million cells were available for testing. Pre-titered amount of monoclonal antibodies were added and lyse-stain-wash protocol was used for staining. Flourochromes used were Fluorescein Isothiocyanate (FITC), phycoerythrin (PE), PerCPCy5.5, PE-Cy7, allophycocyanin (APC) and APC-H7. The prepared tubes were acquired on a BD FACS Canto II flow cytometer (4-2-2 colour configuration) and analysed with BD FACS Diva software version 8.0. The analysis showed blasts with dim expression of Cluster Differentiation (CD) 45 and low side scatter (SSC). They were positive for CD34, CD33, CD117 and MPO along with CD4 and CD11c [Table/Fig-5]. Unfortunately, cytogenetic and molecular testing was not possible as patient refused further evaluation.
Flow cytometry of bone marrow aspirate, Dim cluster differentiation (CD)45+ and low side scatter events gated as blasts (shown in red) were CD34+, CD38+, myeloperoxidase+, CD33+, CD117+, human leukocyte antigen –DR +, CD38+; negative for CD33 and cytoplasmic CD3.
He was advised to undergo low dose cytarabine based chemotherapy. However, he refused therapy and then was lost to follow-up.
Discussion
H-AML represents a small but significant number of patients diagnosed with myeloid malignancies [1,2]. It mainly affects the elderly and accounts for 5-7% of de novo AML. Previous cytotoxic/radiation exposure or therapy needs to be excluded since hypocellular marrows can be seen in cases of therapy-related- AML. H-AML is associated with pronounced cytopenias and <20% bone marrow cellularity [3]. However, in the present case, there was absence of cytopenia in spite of hypocellular marrow indicating preserved normal marrow elements elsewhere in the body.
Diagnostic criteria to distinguish between hypocellular MDS, H-AML or AA have been proposed as there is considerable overlap in clinic-morphologic features [4,5]. The largest study on H-AML reported 123 cases [6]. In rest of the studies only small number of cases has been reported [7-10].
H-AML is termed as a secondary disease in a pre-existing haematologic disorder or prior cytotoxic or radiation therapy and is often misdiagnosed as MDS [5]. It is mostly seen in elderly males as in this report. However, there have been reports of H-AML in children and young adults as well [5,6]. Age and lactate dehydrogenase levels are important prognostic markers [9]. Absence of splenomegaly was notable as this feature differentiates AML arising from a pre-existing primary myelofibrosis [8].
Diagnosis of H-AML depends on diligent morphologic examination (count 100 cells in PBS and 500 cells in BMA), perform iron stain on BMA, assess cellularity in BMB and look for abnormal localization of immature precursors [4]. Although the last feature is not specific, it is more commonly associated with MDS than AML [2]. Features of erythroid dysplasia such as multinucleated forms, Howell-Jolly bodies, nuclear budding or bridging and abnormal ring sideroblasts must caution the pathologist towards the possibility of MDS. Megakaryocytic abnormalities such as multinucleation, hypolobation, abnormal chromatin or nuclear shape, micromegakaryocytes and abnormal clustering also point towards MDS. The single most important feature which distinguishes H-AML from MDS is presence of more than or equal to 20% blasts in an all nucleated cell differential count [4,6]. Neutrophilic hypogranularity or pseudo Pelger-Huet anomalies also exclude AA. The diagnosis may be even more complicated when H-AML is associated with bone marrow necrosis [10]. Concordance rate between pathologists is only 57% when it comes to the diagnosis of H-AML [4]. Only a high degree of clinical suspicion and careful laboratory evaluation will yield correct diagnosis.
Flow cytometry is an invaluable tool in determining Leukaemia Associated Immunophenotype (LAIP) and supplementing evidence for dysplasia in hypocellular marrows [7,8]. When the cellularity of the aspirate is low, concentration procedures such as bulk lysis protocol or Ficoll- Hypaque density gradient centrifugation may be done to ensure adequate number cells available for immunophenotyping. Precautions for rare event analysis must be taken. Appropriate antibody panel design and instrument settings also help in improving sensitivity of the assay. Blasts in AML without maturation have low SSC and moderate CD45 expression and are positive for CD117, CD13, HLA-DR, and often negative for CD34, terminal deoxynucleotidyltransferase (TdT) and sometimes for CD33. Monocytic markers like CD4 and CD11c may be positive. Apart from the lineage specific marker MPO, CD34 is one of the most useful markers for detection of blasts in H-AML. When it is negative, other LAIP such as CD117, lysozyme and CD68 must be used for diagnosis. Flow cytometry is also useful in screening for possible Paroxysmal Nocturnal Haemoglobinuria (PNH) clones which arise in an aplastic setting [5,6]. A high sensitivity PNH assay may incidentally reveal presence of blasts when there is low clinical suspicion of H-AML.
In a major study, 46% of H-AML patients had diploid karyotype or (or –Y) and 28% had chromosome 5 and/or 7 abnormalities [6]. Other abnormalities like t(8;21) [11] and tetraploid clone characterized by two t(15;17) [12] are also described. Any mutation involving KRAS, NRAS, NMP1 and FLT3-ITD may occur in about 2% of patients [6].
The pathogenesis of hypocellularity is still unclear. It is doubtful whether hypocellularity is the primary event which leads to leukemogenesis or a secondary humoral response to leukaemic microenvironment [5]. Recent evidence suggests that outcome of H-AML does not differ from that of non-hypocellular acute myeloid leukaemia [6].
Allogenic stem cell transplantation with supplementation of low dose cytarabine and Granulocyte Colony Stimulating Factor (G-CSF) is the current line of treatment [9]. The dose of cytarabine used in standard AML treatment regimen may not be required for H-AML patients. Treatment with a priming regimen containing homoharringtonine, cytarabine and G-CSF have shown better response rates and improved survival [13]. The improvement with G-CSF based treatment may be due to the increased sensitivity of slowly replicating H-AML cells to G-CSF which either undergo maturation and Apoptosis or killed by cytotoxic effect of improved normal haematopoiesis [14]. Good response rates have been achieved with melphalan based regimen as well specially in H-AML arising from MDS with excess blasts [15].
Conclusion
The diagnosis of H-AML can be difficult and may be confused with hypoplastic MDS and AA. Strict diagnostic criteria must be followed to arrive at correct diagnosis. Presence of dysplasia and abnormal localization of immature precursors may not be able to differentiate between H-AML from H-MDS. Caution must be employed when blast count in BMA is >/=20% even in case of bone marrow hypocellularity. Careful history, diligent peripheral smear examination, adequate BMA and biopsy, detailed morphologic examination, flow cytometry on peripheral blood for detection of PNH clone or blasts as well as flow cytometry on bone marrow aspirate for detection of blasts and asynchronous antigen expression as evidence of myelodysplasia are necessary for accurate diagnosis. Larger prospective multi-centric clinical trials are required for understanding the pathobiology of H-AML and appropriate treatment guidelines.
Compliance with Ethical Standards
Informed consent was obtained from the patient for being included in the study.
[1]. Tuzuner N, Cox C, Rowe JM, Bennett JM, Hypocellular acute myeloid leukaemia: the Rochester (New York) experienceHematol Pathol 1995 9:195-203. [Google Scholar]
[2]. Tuzuner N, Cox C, Rowe JM, Watrous D, Bennett JM, Hypocellularmyelodysplastic syndrome: new proposalsBr J Haematol 1995 91:612-17.10.1111/j.1365-2141.1995.tb05356.x8555063 [Google Scholar] [CrossRef] [PubMed]
[3]. Needleman SW, Burns CP, Dick FR, Armitage JO, Hypoplastic acute leukaemiaCancer 1981 48:1410-14.10.1002/1097-0142(19810915)48:6<1410::AID-CNCR2820480624>3.0.CO;2-4 [Google Scholar] [CrossRef]
[4]. Bennett JM, Orazi A, Diagnostic criteria to distinguish hypocellular acute myeloid leukaemia from hypocellularmyelodysplastic syndromes and aplastic anaemia: recommendations for a standardized approachHaematologica 2009 94:264-68.10.3324/haematol.1375519144661 [Google Scholar] [CrossRef] [PubMed]
[5]. Nagai K, Kohno T, Chen YX, Tsushima H, Mori H, Nakamura H, Diagnostic criteria for hypocellular acute leukaemia: a clinical entity distinct from overt acute leukaemia and myelodysplastic syndromeLeuk Res 1996 20(7):563-74.10.1016/0145-2126(95)00136-0 [Google Scholar] [CrossRef]
[6]. Al-Kali A, Konoplev S, Lin E, Kadia T, Faderl S, Ravandi F, Hypocellular acute myeloid leukaemia in adults: analysis of the clinical outcome of 123 patientsHaematologica 2012 97(2):235-40.10.3324/haematol.2011.04667222058194 [Google Scholar] [CrossRef] [PubMed]
[7]. Mukherjee T, Pramanik S, Dutta R, Acute leukaemia with atypical clinical presentation posing diagnostic dilemmaJ Hematol Blood Transfus Disord 2016 3:01010.24966/HBTD-2999/100010 [Google Scholar] [CrossRef]
[8]. Sehgal T, Naseem S, Kumar N, Varma N, Das R, Ahluwalia J, Hypocellular acute leukaemia: Study of clinical and hematological featuresJournal of Hematopathology 2014 7(4):147-52.10.1007/s12308-014-0219-y [Google Scholar] [CrossRef]
[9]. Park H, Lee J, Choi S, Lee J, Seol M, Lee Y, Hypoplastic acute myeloid leukaemiaBlood 2006 108(11):4493Retrieved from http://www.bloodjournal.org/content/108/11/4493 [Google Scholar]
[10]. Jain D, Singh T, Kumar N, Hypocellular acute myeloid leukaemia with bone marrow necrosis in young patients: two case reportsJournal of Medical Case Reports 2009 3:2710.1186/1752-1947-3-2719171041 [Google Scholar] [CrossRef] [PubMed]
[11]. Purev E, Dumitriu B, Hourigan CS, Young NS, Townsley DM, Translocation (8;21) acute myeloid leukaemia presenting as severe aplastic anaemiaLeukaemia Research Reports 2014 3(2):46-48.10.1016/j.lrr.2014.04.00225003026 [Google Scholar] [CrossRef] [PubMed]
[12]. Kojima K, Imaoka M, Noguchi T, Narumi H, Uchida N, Sakai I, Hypocellular acute promyelocyticleukaemia with a tetraploid clone characterized by two t(15;17)Cancer Genetics and Cytogenetics 2003 145:169-71.10.1016/S0165-4608(03)00097-9 [Google Scholar] [CrossRef]
[13]. Hu X, Fu W, Wang L, Gao L, Lü S, Xi H, HAG regimen improves survival in adult patients with hypocellular acute myeloid leukaemiaOncotarget 2016 7(3):3623-34.10.18632/oncotarget.621126497216 [Google Scholar] [CrossRef] [PubMed]
[14]. Whittle AM, Feyler S, Bowen D, Durable second complete remissions with oral melphalan in hypocellular acute myeloid leukaemia and refractory anaemia with excess blast with normal karyotype relapsing after intensive chemotherapyLeukaemia Research Reports 2013 2:9-11.10.1016/j.lrr.2012.10.00124371768 [Google Scholar] [CrossRef] [PubMed]
[15]. Nimubona S, Grulois I, Bernard M, Drénou B, Godard M, Fauchet R, Complete remission in hypoplastic acute myeloid leukaemia induced by G-CSF without chemotherapy: Report on three casesLeukaemia 2002 16:1871-73.10.1038/sj.leu.240259212200710 [Google Scholar] [CrossRef] [PubMed]