Prenatal Diagnosis of Isolated Type 2 Diastematomyelia at 12 Weeks Using 2D Ultrasound with a Normal Intracranial Translucency
Megha Pandove1, Akhila Vasudeva2, Anjali Mundkur3, Pratap Kumar4
1 Junior Resident, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2 Professor and Unit Chief, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3 Associate Professor, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
4 Professor and Unit Chief, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Akhila Vasudeva, Professor and Unit Chief, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India.
E-mail: akhilavasudeva@gmail.com
First-trimester ultrasound provides an opportunity to screen for various foetal abnormalities. Isolated Diastematomyelia is a rare spinal deformity which is infrequently diagnosed during the first-trimester ultrasound. Here, authors report a case of isolated Type 2 Diastematomyelia which was diagnosed in the 12-week ultrasound during a detailed survey of the foetal spine in view of previous neural tube defects.
Foetal medicine, First-trimester screening, Genetics, Neural tube defect
Case Report
A 25-year-old multigravida, with second-degree consanguineous marriage, underwent routine first-trimester screening in the hospital at 12 weeks. Two of her previous pregnancies were affected by foetal abnormalities, both open neural tube defects. She had a history of foetal losses with recurrent neural tube defects. During her first pregnancy, she delivered a male baby who expired at one month of age due to large meningomyelocele. In second pregnancy, she underwent Medical Termination of Pregnancy (MTP) at 18 weeks of gestation due to foetal thoracic meningomyelocele and gross hydronephrosis. From third pregnancy, she had a healthy three-year-old male child. She underwent Laparoscopic right salpingectomy in her fourth pregnancy in view of tubal ectopic pregnancy. In present pregnancy, there was no antenatal illness or exposure to known teratogens in her. The detailed anatomical survey was performed at 12-weeks along with nuchal translucency. Intracranial translucency was normal. Brainstem and brainstem to occipital distance ratio also appeared normal, but not measured objectively [Table/Fig-1]. However, in the axial view of the lumbar spine, there were extra brightly echogenic foci seen in the posterior aspect of the spinal canal [Table/Fig-2]. There was a widening of posterior ossification centres visible in coronal and axial views along with echogenic spur in the centre [Table/Fig-3,4]. Overlying soft tissues appeared intact [Table/Fig-5]. Features were suggestive of isolated diastematomyelia. The possible good postnatal prognosis was conveyed to the couple. Considering a possible good prognosis, couple decided to continue the pregnancy at this point. Same findings were confirmed at 16-weeks sonography, at this time couple was sent to paediatric orthopaedics for counselling. However, the couple decided on pregnancy termination at this juncture due to financial constraints on paediatric neurosurgery and the need for regular follow-ups in the setting of poor social support. MTP was performed using vaginal misoprostol. Couple consented for a foetal autopsy. Radiographs of the foetus showed widening of the vertebral canal in the lumbar region [Table/Fig-6]. Overlying skin and soft tissue were intact. A foetal autopsy revealed widening of the lumbar vertebral canal [Table/Fig-7,8]. Splitting of the spinal cord was noted in the lumbar region [Table/Fig-9]. The split spinal cord was covered within the single dural sac. There was no evidence of open/closed spina bifida. There were no other structural abnormalities. Brain, neck structures, trachea, oesophagus, heart, major vessels, diaphragm, kidneys, adrenals, stomach, spleen, intestine, rectum and internal genitalia were normal. Hence, the diagnosis of isolated Type 2 diastematomyelia was confirmed.
Intracranial translucency normal. Brainstem and Brainstem to Occipital distance ratio appeared normal;
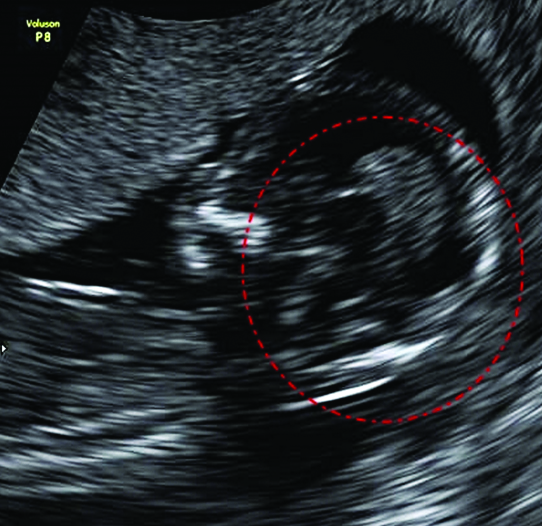
In the axial view of the lumbar spine, there were extra brightly echogenic spur seen in the posterior aspect of the spinal canal.
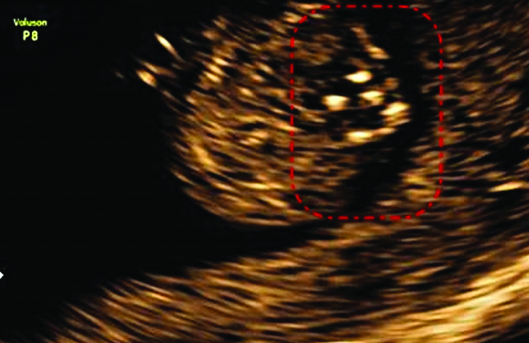
There was a widening of posterior ossification centres visible in coronal and axial views along with echogenic spur in the centre.
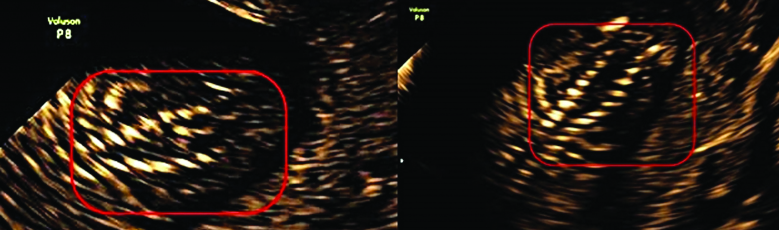
Overlying soft tissues appeared intact;
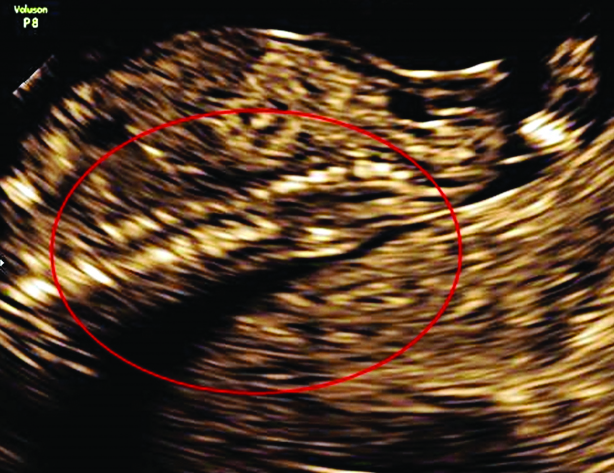
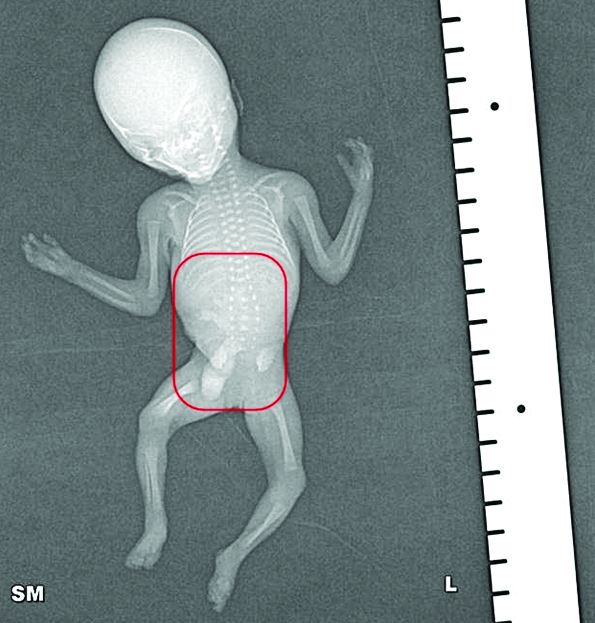
Radiographs of the foetus showed widening of the vertebral canal in lumbar region.
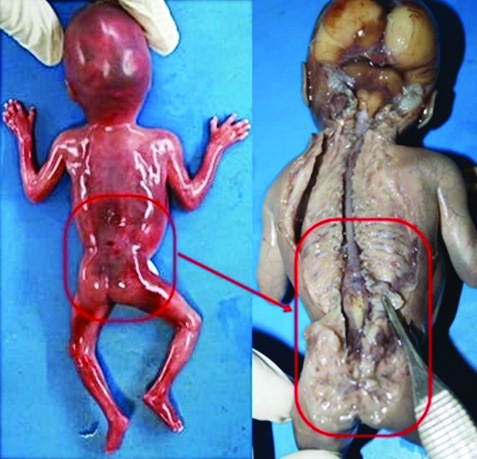
On external examination, there were no obvious anomalies in foetus. Foetal autopsy revealed widening of the lumbar vertebral canal.
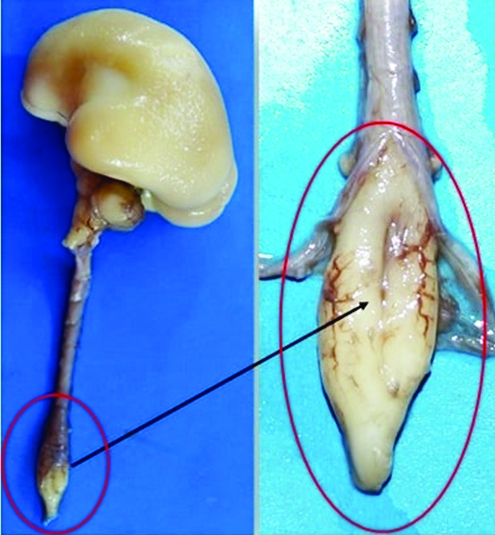
Splitting of spinal cord was noted in lumbar region. The split spinal cord was covered within the single dural sac. There was no evidence of open/closed spina bifida.
Discussion
Routine implementation of the detailed anatomical survey at 11-14 weeks ultrasound has helped in early diagnosis of several foetal abnormalities, including spinal anomalies. Diastematomyelia is a very rare spinal dysraphism with an incidence of 0.06% [1]. It is due to a longitudinal split in the spinal cord, resulting in partial or complete duplication of the spinal cord. The split is caused by an osseous/cartilaginous septum (Type1) or a fibrous septum (Type 2, rarer) tissue in the central portion at the level of the defect in the spinal cord usually at the level of the Lumbosacral region. Diastematomyelia is associated with other malformations in 68.2% of cases [2]. Vertebral abnormality such as hemivertebrae, butterfly vertebrae, kyphosis, spina bifida; Spinal deformity such as meningomyelocele, Arnold-Chiari Type II malformation, neuro schisis; bilateral clubfoot and rarely renal and uterine anomalies have been reported. After birth, 54% skin pigmentation, haemangioma and hair patch are commonly reported to be seen [2]. Median gestational age at prenatal diagnosis has been 17.6±5.2 weeks. Only four cases so far have been diagnosed in the 11-14 weeks scan mostly Type 1 which is easier to diagnose [3-6]. Three of the reported cases had associated craniovertebral anomalies. Authors report prenatal diagnosis of isolated type 2 diastematomyelia at 12 weeks using a targeted 2D Ultrasonography. Split cord malformations are divided into two types based on the dividing septum and dural sac [7] which are:
Type I: duplicated dural sac, with common midline spur (osseous or cartilaginous) and usually symptomatic
Type II: single dural sac containing both Hemicords, with fibrous septum; impairment less marked, rare. In 1985 William and Barth on a 2D scan for a 26-year-old at 23 weeks of period of gestation identified abnormal widening of the posterior ossification centres of the foetal spine with a central, bright linear echo within the canal at the thoracolumbar region in association with an open neural tube defect [8]. An autopsy confirmed the first case of prenatal diagnosis of diastematomyelia and an Arnold-Chiari Type II malformation with resultant hydrocephalus. This is the first known case of Ultrasonographic diagnosis of Diastematomyelia.
Up till 2017, 44 cases have been reported to be diagnosed worldwide prenatally [2]. Mean age at diagnosis was 17 weeks ranging from 12.6 weeks to 36 weeks. Prenatal diagnosis of this spinal defect has been reported late in pregnancy and out of all the cases diagnosed, four cases have been reported to be diagnosed at 11-14 weeks scan in recent few years, mostly Type 1 [3-6]. The most reliable Ultrasonographic feature suggestive of Diastematomyelia is a central echogenic spur with the widening of the spinal canal. Type 2 variety (which was present case), is more likely to be missed as this hyperechogenic focus may be absent or not seen in prenatal sonography. It is said that closed spinal dysraphism, which occurs with diastematomyelia, are more difficult to detect as there are no secondary cranial changes. Present case is also peculiar because there were no other sonological clues in the brain or spine.
Three cases have been seen along with other craniovertebral sonological clues. Alanbay reported a case of thoracolumbar Type I Diastematomyelia in a low-risk primigravida at 14th week [3]. Cranial Examination revealed the presence of a “lemon sign” and “banana sign,” a detailed scan revealed features suggestive of meningocele, thoracic scoliosis, hemivertebrae with classical features of diastematomyelia-thoracolumbar spinal canal dilatation, and extra echogenic foci within the spinal canal. All the findings were confirmed by foetal autopsy, X-Ray, CT, and MRI.
Eleftheriades M et al., diagnosed a Type 1 Diastematomyelia at gestational age 13 weeks 2 days during routine first-trimester screening in a low-risk multigravida [4]. In a midsagittal intracranial translucency view, four lines were present, but the borders of the intracranial translucency were not easily identifiable. Eleftheriades M et al., found increased brain stem diameter and brain stem-to-occipital bone diameter ratio [4]. A careful evaluation of the foetal spine led to the diagnosis of diastematomyelia due to the presence of widening of the vertebral bodies at the lumbar region and the presence of an echogenic area in the spinal canal. This pregnancy was terminated, Postmortem examination confirmed complete Type 1 Diastematomyelia with a cartilaginous diaphragm in the spinal cord and spina bifida occult. This is the first reported association of posterior brain abnormalities in the first trimester with diastematomyelia. So far abnormal four line view has been helpful in the diagnosis of open neural tube defects. However, in present case, intracranial translucency view appeared normal.
Lituania M et al., described the first case of cervicothoracic diastematomyelia and diplomyelia diagnosed at 12.6 weeks of gestation using three-dimensional ultrasound in a low-risk primigravida [5]. No active movements were seen during the scan and only passive foetal movements were observed. Disorganisation of intracranial anatomy and of the ventricular system was noted. Surface rendering by maximum transparency mode identified two hemicords at cervical- thoracic level downwards along with bony/cartilaginous septum suggesting Type 1 diastematomyelia. Omniview and HD Live (virtual fetoscopy) helped to clearly visualise two separate cords. The parents opted for pregnancy termination. An autopsy was refused by the parents.
The fourth reported case is that of an isolated diastematomyelia, the type is not clearly mentioned (mostly Type 1, as it was symptomatic and needed surgery). Has R et al., published a case series of prenatally diagnosed diastematomyelia, in which one of the cases was diagnosed as an isolated diastematomyelia at 13 weeks period of gestation during routine first-trimester screening [6]. The pregnancy was continued and the baby was delivered vaginally at term. Thoracic Diastematomyelia at T3-T4 level was operated upon at five months of age due to the tethered cord. Follow-up till 1 year of age was suggestive of no further neurological or orthopaedic impairment. This case highlights the importance of early diagnosis of isolated diastematomyelia. The wrong diagnosis as an open neural tube defect may lead to unnecessary pregnancy termination. Early prenatal diagnosis leads to appropriate postnatal follow-up that result in timely surgical intervention resulting in a good prognosis. However, in the present case, parents opted for termination despite elaborate counselling on good prognosis. Decisions on pregnancy termination are very specific to the couple, which depends upon their economic situation/social support/past obstetric history.
Conclusion
It is possible to diagnose isolated Type 2 diastematomyelia in a targeted 2D ultrasound between 11-14 weeks. This helps in parental counseling as well as improving postnatal outcome.
[1]. Anderson NG, Jordan S, MacFarlane MR, Lovell-Smith M, Diastematomyelia: diagnosis by prenatal sonographyAJR Am J Roentgenol 1994 163(4):911-14.10.2214/ajr.163.4.80920348092034 [Google Scholar] [CrossRef] [PubMed]
[2]. Wei Q, Cai A, Wang X, Wang X, Xie L, The value of prenatal ultrasonographic diagnosis of diastematomyeliaJ Ultrasound Med 2017 36(6):1129-36.10.7863/ultra.16.0405428304101 [Google Scholar] [CrossRef] [PubMed]
[3]. Karasahin K, Gezginç K, Alanbay I, Ulubay M, Başer I, Ultrasonographic diagnosis of diastematomyelia during the 14th week of gestationTaiwan J Obstet Gynecol 2009 48(2):163-66.10.1016/S1028-4559(09)60279-5 [Google Scholar] [CrossRef]
[4]. Eleftheriades M, Grigoriadis C, Sotiriadis A, Botsis D, Konstantinidou A, Souka AP, Intracranial translucency and spinal cord defects: early prenatal diagnosis of diastematomyeliaJ Ultrasound Med 2013 32(9):1676-84.10.7863/ultra.32.9.167623980231 [Google Scholar] [CrossRef] [PubMed]
[5]. Lituania M, Tonni G, Júnior EA, First trimester diagnosis of cervico-thoracic diastematomyelia and diplomyelia using three-dimensional ultrasoundChilds Nerv Syst 2015 31(12):2245-48.10.1007/s00381-015-2926-926438550 [Google Scholar] [CrossRef] [PubMed]
[6]. Has R, Yuksel A, Buyukkurt S, Kalelioglu I, Tatli B, Prenatal diagnosis of diastematomyelia: presentation of eight cases and review of the literatureUltrasound Obstet Gynecol 2007 30(6):845-49.10.1002/uog.406617726726 [Google Scholar] [CrossRef] [PubMed]
[7]. Pang D, Dias MS, Ahab-Barmada M, Split cord malformation: Part I: A unified theory of embryogenesis for double spinal cord malformationsNeurosurgery 1992 31(3):451-80.10.1227/00006123-199209000-000101407428 [Google Scholar] [CrossRef] [PubMed]
[8]. Williams RA, Barth RA, In utero sonographic recognition ofdiastematomyeliaAJR Am J Roentgenol 1985 144:87-88.10.2214/ajr.144.1.873880632 [Google Scholar] [CrossRef] [PubMed]