Periodontitis is a destructive and progressive inflammatory condition of tooth-supporting tissues, the pathogenesis of which is attributed to several risk factors, including bacteria, the host immune responses and genetics [1]. Although it has been shown that the initiation of periodontal disease is related to periodontal pathogenic bacteria and their products, the extent and severity of tissue destruction is not always correlated with bacterial load [2]. The immune system plays a key role in the host defense and initiation of inflammation and the extent of destruction of periodontal tissues. Recently, a new group of molecules, referred to as important innate immune inflammatory factors, have been introduced and have been named endokines, alarmins or Damage-Associated Molecular Pattern Proteins (DAMPs). Such nomenclature is attributed to the factors released by damaged cells or cells activated under cellular stress conditions. One example of such molecules is S100 phagocytic proteins which are inflammatory response mediators [3]. The first member of S100 protein family was identified as a bovine brain protein by Moore B in 1965 [4]. The term S100 was used due to their solubility in 100% ammonium sulfate solution [5]. Each member of the S100 family is associated with a specific disease based on the gene expression pattern. For example, S100A1 is associated with cardiomyopathy; S100A7 is associated with psoriasis and S100A8, A9 and A12 are associated with inflammatory conditions [5].
S100A12 (calgranulin) was first discovered in the synovial and cerebrospinal fluid of patients with rheumatism, indicating a relationship between A12 and the pathogenesis of chronic and acute inflammatory responses [6]. Recently, a relationship has been shown between S100A12 and periodontitis [7]; S100A12 is released from activated neutrophils and exhibits proinflammatory effects on endothelial and immune cells [8]. There is evidence that S100A12 has chemotactic effects on neutrophils and monocytes [6]. It increases the adhesion of neutrophils and its intravenous injection into rats results in the induction of movement of neutrophils from the bone marrow to the inflammed area [9].
IL-22 is a member of IL-10 cytokine subfamily which is secreted by CD4 memory T cells and NK cells. IL-22 has no known effect on immune cells because none of the resting or activated immune cells express functional IL-22; rather, some non-haematopoietic cells such as skin keratinocytes and the epithelial cells of the digestive and respiratory systems express IL-22 receptors and are the possible targets of this cytokine [10].
Based on recent reports, Th17 is the most important cell to secrete IL-22 and the most important factor in the pathogenesis of cell-mediated tissue injury, which might be due to auto-immunity of the immune response against microbial infection. Recent studies, based on higher levels of expression of IL-17 and IL-23 in periodontal lesions compared to the control group, have shown the stimulation of Th17 pathway by IL-23 in inflammatory periodontal lesions. Therefore, it is possible that IL-22 has a role in the pathogenesis of periodontal disease [10].
IL-17 and IL-22 have a synergistic effect on increasing the expression of antimicrobial peptide genes by keratinocytes such as B-defensin 2,3 and S100A7/8/9 [11] and S100/calgranulin induces the synthesis of IL-22 [12].
Some studies have shown a positive relationship between the synthesis of IL-22 and the severity of chronic periodontitis; however, some other studies have not shown a relationship between the expression of this gene and periodontal diseases [1,2,13,14]. In addition, due to the limited number of studies carried out on S100A12 in patients with chronic periodontitis, its role as a protective or destructive factor is not clear. Therefore, the present study was undertaken to evaluate the effect of phase I periodontal treatment on the concentrations of IL-22 and S100A12 in patients with chronic periodontitis, referring to the Faculty of Dentistry, Shahed University.
Materials and Methods
The present clinical trial lasted for 12 months during 2015-2016, and a total of 22 patients (14 female and 8 male), with a mean age of 39.55±10.25 years, were selected from those referred to the Department of Periodontics, Faculty of Dentistry, Shahed University. The protocol of this research work was approved by the Ethics Committee of Shahed University and registered in Iranian Registry of Clinical Trials. (IRCT2016123131687N1).
The inclusion criteria consisted of the presence of at least 20 teeth in the oral cavity and affliction with moderate and severe chronic periodontitis (CAL ≥3 mm, PD ≥5 mm) with radiographic evidence of bone loss. The exclusion criteria consisted of patients with cardiovascular diseases and hypertension, use of tobacco, pathologic conditions in the oral cavity, subjects with chronic inflammatory conditions such as rheumatoid arthritis, presence of tumors, pregnancy, use of any medications affecting the periodontium, presence of systemic diseases that affect the course of periodontal disease, having undergone scaling and root planing during the previous six months and a history of taking antibiotics and NSAIDs during the previous three months.
According to the standard deviations which were observed in similar works and sample sizes in other similar studies [7], the sample size was estimated as n=22. The power of the study was 80%. The subjects in the test group consisted of patients with chronic periodontitis. The non-random sampling technique was used to select subjects from the available individuals.
The aim of the study was thoroughly explained to the subjects and informed consent forms were obtained from all the subjects. The subjects were interviewed privately to collect personal and medical data, followed by periodontal examinations. One trained and calibrated last-year postgraduate student in periodontology carried out all the clinical and periodontal examinations. The clinical parameters that were recorded consisted of probing depth and clinical attachment levels on 6 surfaces of the teeth (mid-buccal, mid-lingual, mesiobuccal, mesiolingual, distobuccal, distolingual) with the use of a hand periodontal probe (Offset Williams Probe, Hu-Friedy, Chicago, IL).
The Measuring Technique
Samples were taken from two areas in each individual. These two areas exhibited the highest CAL and signs and symptoms of inflammation and radiographic examination confirmed bone loss. Gingival crevicular fluid samples were collected from the gingival sulcus or the pocket with the use of Perio papers (Smithtown, NY 11787 Oraflow). The paper strips were placed in the gingival sulcus in proximal areas and left in place for 30 seconds. The paper strips were then retrieved and visually examined carefully. Tapes contaminated with saliva, blood, pus or microbial plaque were excluded and sampling was repeated. At this stage, the Periostrips were placed within microtubes and stored within a freezer at -20°C, and then sent to the laboratory.
Phase I Treatment
After collecting samples from the test group subjects, phase I periodontal treatment was rendered, which consisted of scaling and root planing and oral hygiene control. The subjects were recalled after four weeks for collecting samples again. The same areas underwent sampling again to collect GCF samples using the same procedures.
GCF Processing and Enzyme-Linked Immunosorbent Assay (ELISA) for the quantitation of IL-22 and S100A12 in GCF samples
In the laboratory, 50 μL of Phosphate-Buffered Saline (PBS) buffer were added into the microtubes which were centrifuged at 3000 rpm for 15 minutes. Then, the microtubes were placed in a refrigerator at -70°C for 24 hours. A commercial ELISA kit (Human S100B: Biovendor, Human IL-22: eBioscience) was used to determine the concentrations of cytokines in the GCF. The ELISA test was done according to the manufacturer’s instruction in the catalogue.
The IL-22 plate was rinsed with 400 μL of the buffer twice before being used. Then 100 μL of the standard solution was prepared; 50 μL of the sample were added to the wells and incubated at 400 rpm for 2 hours. Subsequently, 100 μL of conjugated biotin solution were added to all the wells and incubated at room temperature at 400 rpm for 1 hour. Then 100 μL of streptavidin-HRP solution was added to all the cavities and incubated at room temperature at 400 rpm for 1 hour. Subsequently, 100 μL of TMB solution were added to each well and incubated in a dark environment for 10 minutes. Finally, 100 μL of stop solution were added and the concentrations were determined with the use of an ELISA reader at a wavelength of 450 nm.
The S100A12 plate was rinsed with 400 μL of the buffer solution twice before being used. Then 100 μL of the standard solution were prepared and 50 μL of the sample were added to each well, followed by incubation at 300 rpm for 2 hours. Then 100 μL of conjugated biotin solution (11/88 μL of assay buffer+0.12 μL of conjugated biotin) were added to all the wells and incubated at room temperature at 300 rpm for 1 hour. Subsequently, 100 μL of streptavidin-HRP solution were added and incubated at room temperature at 300 rpm for half an hour. A total of 100 μL of substrate solution were added to each well and incubated for 15-20 minutes in a dark environment. Then, the reaction was stopped by adding 100 μL of the stop buffer solution, followed by determining S100A12 concentration with an ELISA reader at a wavelength of 450 nm.
Then, means and standard deviations of PD and CAL and parameters related to the concentrations of IL-22 and S100 before and after the treatment were compared.
Statistical Analysis
Kolmogorov-Smirnov test was used to evaluate normal distribution of data. The study groups were compared with the use of Wilcoxon’s signed-rank test. Spearman’s test was used to evaluate correlation between the concentrations of S100A12 and IL-22 before and after treatment in patients with chronic periodontitis by considering CAL and PD. Finally, the concentration of S100 before treatment with CAL and PD, correlation between concentration of S100 after treatment with CAL and PD, concentration of IL-22 before treatment with CAL and PD and concentration of IL-22 after treatment with CAL and PD was analysed. Statistical significance was set at p≤0.05. All the statistical analyses were carried out with SPSS version 21.0.
Results
In the present study, the effect of phase I periodontal treatment on the concentrations of IL-22 and S100A12 was evaluated in patients with chronic periodontitis. The mean and standard deviation of subjects’ age was 39.55±10.25 years, of 22 subjects, 14 and 8 were female and male, respectively.
The means and standard deviations of PD before and after treatment were 5.54±1.30 and 4.40±1.34 mm, respectively. The means and standard deviations of CAL before and after treatment were 5.62±1.70 and 4.36±1.59 mm, respectively. [Table/Fig-1] presents the descriptive statistical parameters related to the concentrations of IL-22 and S100 before and after treatment separately.
Descriptive statistical parameters related to the concentration of IL-22 and S100 before and after treatment separately.
| Statistical parametersVariable | Mediator | Mean | Standard deviation | Minimum | Maximum | Number |
|---|
| Before treatment | IL-22 | 0.760 | 0.009 | 0.06 | 0.1 | 22 |
| S100A | 0.283 | 0.418 | 0.04 | 1.83 | 22 |
| After treatment | IL-22 | 0.750 | 0.009 | 0.06 | 0.1 | 22 |
| S100A | 0.197 | 0.225 | 0.4 | 0.87 | 22 |
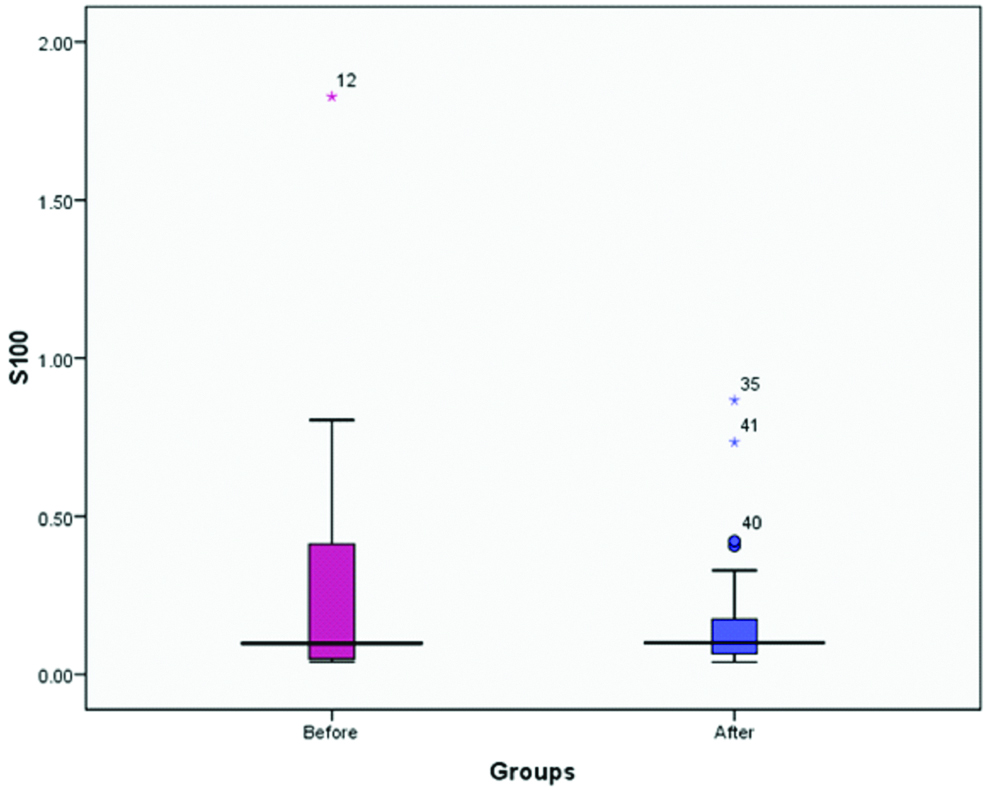
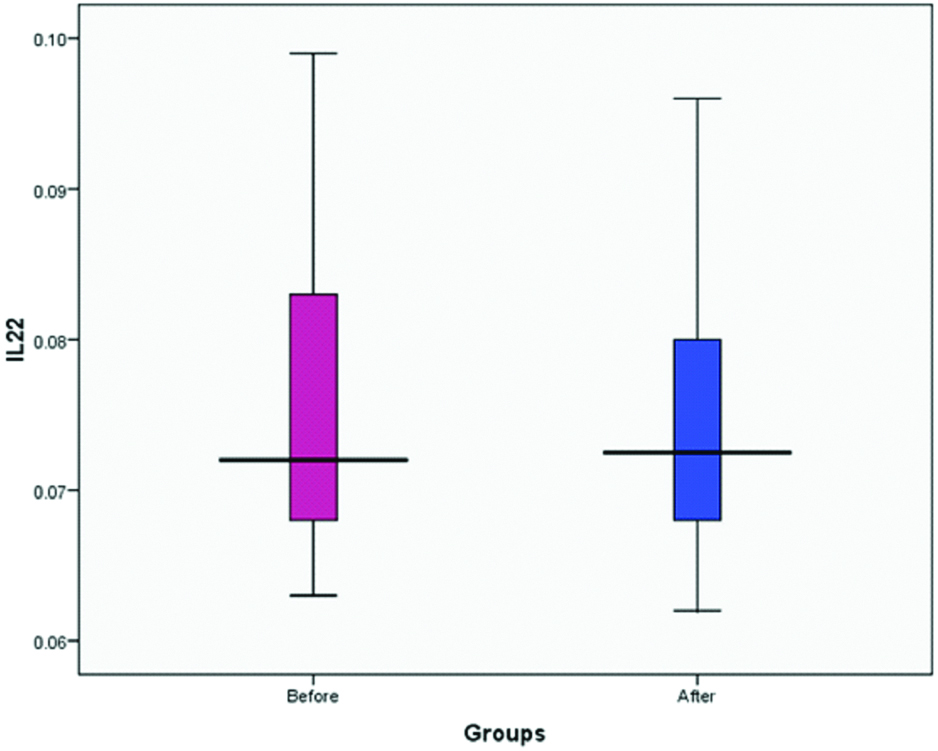
[Table/Fig-2,3] present the concentrations of IL-22 and S100 before and after treatment, respectively, with the use of Wilcoxon’s signed-rank test.
Concentration of IL-22 before and after treatment respectively, with the use of Wilcoxon’s signed-rank test.
| | Number | Average Rating | Total Ratings | Z | p-value |
|---|
| IL-22. After-IL-22. Before | Negative Ratings | 12a | 8.83 | 106.00 | -0.443 | 0.658 |
| Positive Ratings | 7b |
| Equal Ratings | 3c | 12.00 | 84.00 |
| Total | 22 |
a. IL-22. After < IL-22. Before; b. IL-22. After > IL-22. Before; c. IL-22. After = IL-22. Before
Concentration of S100 before and after treatment respectively, with the use of Wilcoxon’s signed-rank test.
| | Number | Average Rating | Total Ratings | Z | p-value |
|---|
| S100. After-S100. Before | Negative Ratings | 10a | 12.70 | 127.00 | -0.016 | 0.987 |
| Positive Ratings | 12b |
| Equal Ratings | 0c | 12.70 | 127.00 |
| Total | 22 |
a. S100. After < S100. Before; b. S100. After > I S100. Before; c. S100. After = S100. Before
As shown in the [Table/Fig-2], phase I treatment of chronic periodontitis did not result in a significant difference in the concentration of IL-22 in the gingival crevicular fluid, despite the differences in the means. Based on the [Table/Fig-3], phase I treatment did not result in a significant difference in the concentration of S100 in gingival crevicular fluid. [Table/Fig-4,5] show comparisons between mean concentrations of IL-22 and S100, respectively.
Comparison between mean concentrations of S100 before and after treatment.
Comparison between mean concentrations of IL-22 before and after treatment.
Spearman’s correlation coefficient did not reveal any correlation between IL-22 and S100 concentrations before treatment (r=0.027, p≈0.906). In addition, no such correlation was found between these two variables after treatment (r=0.298, p≈0.178).
Regarding the correlation between the concentrations of IL-22 before and after treatment (r=0.171, p≈0.446) and concentrations of S100 before and after treatment (r=0.360, p≈0.100) and the clinical parameters (mean PD and mean CAL before and after treatment), there was an inverse correlations between the mean PD and S100 before treatment (p<0.05). In relation to CAL, although an inverse correlation was expected between CAL and S100 concentration, no significant correlation was found between them (r=-0.382, p≈0.079). There was an inverse correlation between S100 concentration and mean PD before treatment, i.e., there was a decrease in PD with an increase in S100 concentration (r=-0.490, p≈0.021). Despite an inverse correlation between S100 concentration and mean CAL before treatment, the correlation was not statistically significant (p<0.1) [Table/Fig-6,7].
Correlation between the concentrations of S100 before treatment and mean PD with Spearman’s test.
| Spearman Correlation Coefficient | Mean. PD Before | S100 Before |
|---|
| Mean. PD Before | Correlation Coefficient | 1.000 | -0.490* |
| p-value | | 0.021 |
| Number | 22 | 22 |
| S100 Before | Correlation Coefficient | -0.490* | 1.000 |
| p-value | 0.021 | |
| Number | 22 | 22 |
Correlation between the concentrations of S100 before treatment and mean CAL with Spearman’s test.
| Spearman Correlation Coefficient | S100 | Mean. CAL |
|---|
| S100 | Correlation Coefficient | 1.000 | -0.382 |
| p-value | | 0.079 |
| Number | 22 | 22 |
| Mean. CAL | Correlation Coefficient | -0.382 | 1.000 |
| p-value | 0.079 | |
| Number | 22 | 22 |
The extent of decrease in PD and CAL was calculated and their correlation with S100 and IL-22 concentrations before and after treatment were determined. The results showed that only the concentration of S100 after treatment, had an inverse correlation with PD. In other instances, no significant correlation was found.
There was a significant and inverse correlation between concentration of S100 after treatment and the amount of decrease in PD after phase I treatment (r=-0.646, p=0.004). There was an inverse and significant correlation between the concentration of S100 after treatment and the decrease in CAL after phase I treatment (r=-.500, p=0.035) [Table/Fig-8,9].
Correlation between the concentrations of S100 after treatment and mean PD with Spearman’s test.
| Spearman Correlation Coefficient | PD Reduction After Treatment | S100. After |
|---|
| PD Reduction After Treatment | Correlation Coefficient | 1.000 | -0.646** |
| p-value | | 0.004 |
| Number | 22 | 22 |
| S100. After | Correlation Coefficient | -0.646** | 1.000 |
| p-value | 0.004 | |
| Number | 22 | 22 |
Correlation between the concentrations of S100 after treatment and mean CAL with Spearman’s test.
| Spearman Correlation Coefficient | CAL Reduction After Treatment | S100. After |
|---|
| CAL Reduction After Treatment | Correlation Coefficient | 1.000 | -0.500* |
| p-value | | 0.035 |
| Number | 22 | 22 |
| S100. After | Correlation Coefficient | -0.500* | 1.000 |
| p-value | 0.035 | |
| Number | 22 | 22 |
The correlation between concentration of IL-22 before treatment and PD (r=0.308, p≈0.164), concentration of IL-22 before treatment and CAL (r=0.209, p≈0.350), the concentration of IL-22 after treatment and the amount of decrease in PD after phase I treatment (r=0.0.085, p≈0.708) and the concentration of S100 after treatment and the decrease in CAL after phase I treatment (r=-0.500) (r=0.0.247, p≈0.267) was not significant.
Discussion
The results of the present study did not reveal a significant difference between the concentrations of IL-22 and S100 before and after phase I periodontal treatment.
IL-22 is a member of IL-10 cytokine family. It is an important mediator for the induction of cellular and innate immune responses. One of its important effects is stimulation of mucous epithelial cells to combat bacteria [15,16]. Menegat JSB et al., reported that the concentration of IL-22 in gingival tissues of patients with periodontitis was much higher than that in intestinal tissue samples of patients with Inflammatory Bowel Disease (IBO). They reported that IL-22 is associated with IL-23 and IL-6 [17].
Isaza-Guzmán DM et al., reported that the concentration of IFN-γ and the IFN-γ/IL-22 ratio were higher in patients with chronic periodontitis compared to healthy subjects [1]. Amini Behbahani A et al., reported no expression of IL-22 gene in healthy subjects and those with chronic periodontitis [2], Kato-Kogoe N et al., reported that the gingival tissue in comparison to periodontal ligament has no capacity to express IL-22 or its expression in the gingival tissue is at a very low level [10].
Differences in the methodologies of studies are one of the most important reasons for differences between the results of the present study and the study above. In the present study, the concentrations of IL-22 were evaluated before treatment and after phase I treatment of chronic periodontitis and we did not intend to compare periodontitis with other cases. In addition, different measurement techniques and different sample sizes were used. The lack of significant differences between the concentration of IL-22 before and after treatment might be attributed to the low concentrations of these cytokines and also to the fact that the gingival tissue is very weak in relation to the synthesis of IL-22, which in itself affects the decrease in IL-22 concentration in the GCF.
Hosokawa Y et al., reported that IL-22 induces the synthesis of CXCL-10 in human gingival fibroblasts [18]. Since CXCL-10 has a chemotactic effect on T helper 1 cells which are not very active in periodontitis, a decrease in T helper 1 cell counts in periodontitis might reflect a decrease in the secretion of IL-22.
In relation to the concentration of S100, too, there was no significant difference in the concentration of this cytokine before and after phase I periodontal treatment. S100 is a family of proteins with low molecular weight in vertebrates, with a role in the regulation of cellular activity, cellular skeleton, enzymatic activity, growth and differentiation of cells and inflammatory responses [19,20]. Mah SJ et al., reported that bone destruction in periodontitis might be associated with an increase in the expression of S100A4 in PDL cells [21].
Pradeep AR et al., reported that S100A12 and CRP (C-reactive protein) can be considered as two parameters for inflammatory activity in GCF and serum of patients with periodontal disease [7]. Heo SH et al., reported that S100A2 is a functional component in immune responses in periodontitis and can be considered a potential parameter for periodontitis [5].
Kojima T et al., discovered S100A8 and S100A9 in the gingival crevicular fluid and reported that these two members of S100 family might be considered a marker for periodontal disease [22]. The difference in the results might be attributed to differences in study methodologies. Therefore, the results of the present study cannot be considered contrary to the results of other studies. In addition, in studies carried out on GCF samples it was not clear in what stage periodontitis was and whether the lesions were active or inactive.
In addition, the results of the present study showed no significant correlation between S100 and IL-22. Chellan B et al., reported that S100/calgranulininduces the secretion of IL-22 [12]. In other words, the reason for differences might be the low concentrations of S100A in the present study, which resulted in its inability to induce the secretion of IL-22.
In relation to the correlation between the concentrations of S100 and IL-22 and the clinical parameters, there was a significant and inverse correlation only between the concentration of S100 and the mean PD before treatment. In relation to the correlation between S100 and CAL, although an inverse correlation was expected between these two variables, statistical analyses showed that the correlation was not significant. However, there was an inverse correlation between CAL and S100; and PD and S100 after the treatment. Since no such study has been carried out to date, these results cannot be compared with those of other similar studies.
Limitation
The limitations of our study were lack of ELISA kits in our country and some of the samples were excluded because the absorption of GCF by the Perio strips was not sufficient. The result of present study can be useful in early diagnosis of periodontitis. Also, these markers may be useful to monitor the treatment of periodontitis.
Conclusion
Overall, it was concluded from the results of the present study that possibly the periodontal tissues are weak cellular sources for the secretion of IL-22 and S100. Given the inverse correlation between the concentration of S100 and the mean PD before treatment and also the inverse and significant correlation between the concentration of S100 and a decrease in CAL and PD after treatment, a possible protective role might be considered for S100 in the incidence of periodontitis and the success of phase I treatment for chronic periodontitis. A significant difference in the concentrations of these two molecules could be achieved by evaluation of the periodontal surgery.
In this context, it might be possible to witness the synergistic effect of IL-22 and S100 on prevention of the progression of periodontal disease by induction of T helper 1 cell responses. Therefore, in advanced cases, due to the dominance of T helper 2 cell responses, a decrease in the concentration of these two cytokines is expected. However, further studies are necessary to substantiate the above hypotheses.
a. IL-22. After < IL-22. Before; b. IL-22. After > IL-22. Before; c. IL-22. After = IL-22. Before
a. S100. After < S100. Before; b. S100. After > I S100. Before; c. S100. After = S100. Before