Dental caries is a worldwide phenomenon defined as the result of a localised chemical dissolution of the tooth surface caused by acid production by the dental plaque biofilm which occurs as a result of persistent exposure to sugars [1]. It is an age old dental phenomenon that has inflicted much harm to the human race throughout history [2]. Teenagers acquire the disease early in childhood and reach a chronic stage at adult life [3]. A vastly diverse category of microorganisms are none other than oral streptococci [4] which are also the predominant pioneer species colonising the oral cavity [5]. Although existing in a symbiotic environment, the oral streptococci are pathogens that are highly capable of giving rise to a host of other diseases pertaining to brain and liver abscesses including infective endocarditis [6]. Clarke JK isolated streptococci from human caries and named it Streptococcus mutans. The pathological process of dental caries is attributed to the ever intense activity of Streptococcus mutans based on microbiological culture interpretation from shallow carious lesions [7]. The key feature in the establishment of a cariogenic dental plaque is the interplay within the dental biofilm [8]. However, loss of tooth structure is attributed to the long process of interaction between host, diet, microbes and time [9].
The prevention and treatment of dental caries is entirely focused upon reducing the oral microflora, maintaining an alkaline environment and to provide appropriate diet counselling thereby, ensuring a safe, effective and predictable outcome [10]. A non-invasive approach in the form of mouthwashes, gels, varnishes, chewing gums can be administered to limit the spread of cariogenic microorganisms.
Mouthwashes, in addition to mechanical plaque control measures have been found to be very effective in reducing the oral microbial load. Chlorhexidine mouthwashes are the most effective chemical method in order to minimise plaque accumulation [11]. In the management of dental caries, chlorhexidine has been limited to high caries risk patients [12]. The literature relating to the use of chlorhexidine is immense; the proof of the agent’s efficacy in such a role is beyond dispute. The disadvantages of chemical mouthwashes includes staining of teeth, altered taste perception, metallic taste, burning sensation etc., [13]. The capacity of chlorhexidine to prevent and control caries by its antimicrobial effect, has been controversial and the evidence is still inconclusive [14].
Although fluoride being anti-cariogenic and chlorhexidine, a broad-spectrum anti-microbial agent are effective to varying degrees, however, the long term use of the latter can spark an undesirable transformation of the normal oral and intestinal flora [15]. Tolerance of bacteria and development of stains in addition to vomiting and diarrhoea has been well documented in the past in accordance with the use of the aforesaid antimicrobials [16,17]. The threat of fluorosis in many countries with the frequent use of fluoride containing products has also led to the notion that fluoride alone as a therapeutic agent cannot be banked upon [18].
In contrast, natural products have been proven to be safe, consisting of biologically active compounds which may have potential therapeutic uses in dentistry. Polyphenolic compounds, cited to be the most potent of all natural products attributed to its credible anti-caries actions are derived from diverse food, beverages, traditional herbs. Natural products have been widely studied to accomplish preventive strategies against dental caries including the control of cariogenic bacteria within the dental plaque biofilm and enhancement of tooth remineralisation and to assist in the resistance to demineralisation [19].
The aim of this systematic review was to assess whether the anti-bacterial effects of natural product containing mouthwash is superior to the gold standard chlorhexidine mouthwash against Streptococcus mutans.
Do natural products bring about significant removal of Streptococcus mutans from saliva compared to chlorhexidine?
Intervention- Cacao bean husk, cranberry, neem, propolis, green tea, black tea, oolong tea, licorice, Terminaliachebula, emblicaofficinalis, Terminaliabellerica, triphala, pomegranate, tulsi, shiitake mushroom, bloodroot, babool, ginger.
Comparison- Chlorhexidine compared with natural products mouthwash.
Outcome - Reduction in streptococcus mutans.
Materials and Methods
The Institutional Review Board Number was SRB/MDS/ENDO/17-18/0022.
Sources Used
For recognition of the various studies included or considered for this review, detailed search strategies were developed for the database searched.
Searched Databases
The following databases were searched: PubMed, PubMed Advanced Search, Science direct, Cochrane Database of Systematic Reviews, LILACS, Google scholar until December 2017.
PubMed search strategy Search
(((((((((((((((((((caries, dental {MeSH Terms}) OR cervical caries {MeSH Terms}) OR dental caries) OR arrested caries) OR proximal caries) OR enamel caries) OR white spot lesion) OR dental lesion) OR acute caries) OR carious teeth) OR humans) OR adults) OR high caries risk) OR dental decay) OR ((decayed, missing, and filled teeth {MeSH Terms})))) AND ((((((((((((((((((((((((((((((((((((((((((herbal mouthwash) OR natural products mouthwash) OR herbal mouthrinse) OR natural products mouthrinse) OR plant extracts mouthwash) OR plant extracts mouthrinse) OR cacao bean husk extract mouthwash) OR CBHE mouthwash) OR cranberry mouthwash) OR garlic mouthwash) OR neemmouthrinse) OR neem mouthwash) OR propolis mouthwash) OR propolismouthrinse) OR Azadirachtaindica mouthwash) OR Azadirachtaindicamouthrinse) OR green tea mouthwash) OR green tea mouthrinse) OR black tea mouthwash) OR black tea mouthrinse) OR oolong tea mouthwash) OR Camellia sinensis mouthwash) OR Camellia sinensismouthrinse) OR licorice mouthwash) OR triphala mouthwash) OR triphalamouthrinse) OR Terminaliachebula mouthwash) OR Terminaliachebulamouthrinse) OR Terminaliabellerica mouthwash) OR Terminaliabellericamouthrinse) OR Emblicaofficinalis mouthwash) OR Emblicaofficinalismouthrinse) OR pomegranate peel extract mouthwash) OR pomegranate mouthrinse) OR pomegranate mouthwash) OR Punicagranatum mouthwash) OR shiitake mushroom mouthwash) OR bloodroot mouthwash) OR tulsi mouthwash) OR tulsimouthrinse) OR baboolmouthrinse) OR ginger mouthwash) OR chitosan mouthwash) OR Acacia nilotica mouthwash)) AND ((((chlorhexidine mouthwash) OR 0.2% chlorhexidine mouthwash) OR chlorhexidinemouthrinse) OR 0.2% chlorhexidinemouthrinse)) AND ((((((((((antibacterial) OR antimicrobial) OR anticariogenic) OR colony count) OR colony forming units) OR Streptococcus mutans) OR S.mutans) OR S.mutans reduction) OR streptococcus mutansreduction) OR microbial count)) AND ((((((in vivo) OR randomized clinical trial) OR randomized controlled clinical trial) OR clinical trial) OR in vivo study) OR clinical study)
Language: Studies in English language were selected.
Hand Search: The following journals were hand searched:
Caries research
Journal of Conservative dentistry
European journal of dentistry
Journal of Dentistry
Eligibility Criteria
Inclusion Criteria
Types of Studies: The type of studies included in this systematic review were in vivo studies, randomised clinical trials, comparative clinical trials, prospective clinical trials in which natural products and chlorhexidine mouthwash have been compared, studies in which population assessed were adults.
Types of Participants: Patients between 18-70 years of age with dental caries.
Types of Interventions: Natural products mouthwash and chlorhexidine mouthwash.
Types of Outcome Measures: The outcome variable selected was the antibacterial efficacy of natural products mouthwash and chlorhexidine mouthwash against Streptococcus mutans.
Exclusion Criteria
The type of studies excluded from this systematic review were in vitro studies, animal studies, studies in which the population assessed were children, studies not comparing chlorhexidine as gold standard.
Publication Status of the Selected Articles
All the articles included in this systematic review were published studies. The screening of the selected articles were first done based on title scan after which the abstract was examined, following which full-text articles were obtained and reviewed. Decision to select the articles for this systematic review was solely based on whether the articles strictly followed the eligibility criteria or not.
Results
Description of Studies
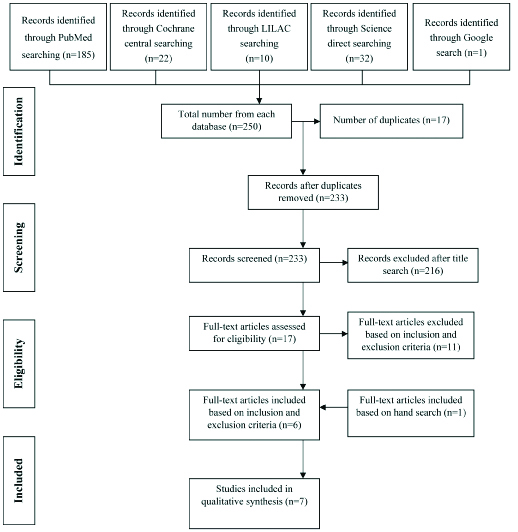
The search identified 250 publications out of which 17 duplicates were removed and 216 were excluded. Full articles were procured for 17 studies and evaluated. After evaluation, 11 of these publications were excluded based on inclusion and exclusion criteria, after reading the full text article [Table/Fig-1] [20-30]. Finally, 6 were included based on the aforesaid criteria. Following hand search, 1 article was included as it satisfied the inclusion and exclusion criteria. Therefore, a total of 7 publications fulfilled all criteria for inclusion. [Table/Fig-2] shows the search flowchart. General information of the selected articles are given in [Table/Fig-3] [4,10,31-35].
Characteristics of excluded articles [20-30].
| S. No | Author | Year | Reason for Exclusion |
|---|
| 1 | Santiago KB et al., [20] | 2017 | Reduction of S.mutans not evaluated |
| 2 | Jain I et al., [21] | 2016 | Adult population not compared |
| 2 | Umar D et al., [22] | 2016 | Adult population not compared |
| 4 | Darvishi HK et al., [23] | 2013 | Reduction of S.mutans not evaluated |
| 5 | Balappanavar AY et al., [24] | 2013 | Reduction of S.mutans not evaluated |
| 6 | Baradari AG et al., [25] | 2012 | Reduction of S.mutans not evaluated |
| 7 | Ramalingam K et al., [26] | 2012 | In-vitro study |
| 8 | Asokan S et al., [27] | 2008 | Adult population not compared |
| 9 | Weintraub JA et al., [28] | 2005 | In-vitro study |
| 10 | Vanka A et al., [29] | 2001 | Adult population not compared |
| 11 | Gultz J et al., [30] | 1998 | Adult population not compared |
General information of selected articles [4,10,31-35].
| S. No | Author | Year | Country | Study Design | Sample Size | Set-Up | Techniques Used | Method of Evaluation |
|---|
| 1 | Botelho MAet al., [31] | 2009 | Brazil | Randomised clinical study | n=55Group 1 (n=27) – Essential oilGroup 2(n=28) – 0.12% chlorhexidine | University | Quantitative analysis using microbial culture method (Colony forming units) | Fisher’s-exact test and Mann-Whitney U-test, Wilcoxon test |
| 2 | Srinagesh J et al., [32] | 2011 | India | Randomised controlled trial | n=57Group 1 (n=18) – 6% triphalaGroup 2(n=19) – 0.2% chlorhexidineGroup 3 (n=20) – Plain water | University | Quantitative analysis using microbial culture method (Colony forming units) | ANOVA, post-hoc test |
| 3 | Srinagesh J et al., [4] | 2012 | India | In vivo study | n=60Group 1 (n=20) – 6% triphalaGroup 2(n=20) – 0.2% chlorhexidineGroup 3 (n=20) – Plain water | University | Quantitative analysis using microbial culture method (Colony forming units) | ANOVA, post hoc test |
| 4 | Velmurugan A et al., [10] | 2013 | India | In vivo study | n=45Group 1 (n=15) - 20% aqueous extract of T. chebulaGroup 2 (n=15) - 20% aqueous extract of E. officinalisGroup 3 (n=15) – 0.2% Chlorhexidine | University | Quantitative analysis using microbial culture method (Colony forming units) | One-wayANOVA, Post-hoc Tukey test. |
| 5 | Khairnar MRet al., [33] | 2015 | India | Randomised clinical trial | n=50Group 1 (n=25) – ChlorhexidineGroup 2 (n=25) – Cranberry | University | Quantitative analysis using microbial culture method (Colony forming units) | Paired t-test for intragroup comparison for evaluation of streptococcal CFU count, unpaired t-test for intergroup comparison for difference in reduction |
| 6 | Yadav M et al., [34] | 2017 | India | Randomised controlled trial | n=45Group 1 (n=15) – 2% Green coffee bean extractGroup 2 (n=15) – 0.2% ChlorhexidineGroup 3 (n=15) – Sterile water | University | Quantitative analysis using microbial culture method (Colony forming units) | One-way ANOVA, Paired t-test for intragroup comparison, Post-hoc test. |
| 7 | Usha C et al., [35] | 2017 | India | Randomised clinical trial | n=46Group (n=23) – 0.12% chlorhexidineGroup (n=23) – Stevia mouthwash | University | Quantitative analysis using microbial culture method (Colony forming units) | Wilcoxon matched-pairs signed-rank test for intergroup comparison, Mann-Whitney test for intragroup comparison. |
Interpretation of Results
Mostly systematic reviews will perform meta-analysis, which involves the statistical pooling of data from individual studies when the studies are similar. A meta-analysis can yield a more precise overall estimate of the treatment effect. However, meta-analysis may not be appropriate in many situations. Owing to the heterogeneity among the studies such as difference in the mouthwash types, sample sizes and follow-up periods, we could not perform a meta-analysis to summarise the data of included studies. Hence, only descriptive evaluation of data has been provided.
Seven Clinical studies fulfilled the criteria for being included in this review [4,10,31-35].
In the present systematic review, of the seven articles reviewed, two were in vivo studies, two were randomised controlled trials and three were randomised clinical trials which compared the antibacterial efficacy of natural products and chlorhexidine mouthwash by quantitative analysis using microbial culture method (Colony forming units) [Table/Fig-3] [4,10,31-35].
The natural products used in each study were Essential oil, Triphala, Terminaliachebula, Emblicaofficinalis, Stevia rebaudiana, Cranberry and Green coffee bean.
Botelho MA et al., evaluated and compared the antibacterial efficacy of an essential oil mouthwash comprising of L.sidoides with that of chlorhexidine [31]. It was clearly shown that both the groups exerted a 58% reduction in Streptococcus mutans count at the end of seven days [Table/Fig-4].
Outcome in the included studies [4,10,31-35].
| S. No | Author and Year | Materials used | Evaluation period | Mean values (Percentage reduction in S.mutans) | Outcome |
|---|
| Chlorhexidine | Natural products and control |
|---|
| 1 | Botelho MA et al., 2009 [31] | Group 2: Chlorhexidine | Group 1: L.sidoides | On 1st day and after 7 days | Group 1: 58%Group 2: 58% | No significant difference between the two groups. |
| 2 | Srinagesh J et al., 2011 [32] | Group 2: Chlorhexidine | Group 1: 6% triphalaGroup 3: Passive control group | On 1st, 15th and 45th day | At 15 daysGroup 1: 83%Group 2: 80%Group 3: 3%At 45 daysGroup 1: 67%Group 2: 65%Group 3: -7% | Group 1 and 2 differed significantly from control. No significant difference between group 1 and group 2. |
| 3 | Srinagesh J et al., 2012 [4] | Group 2: Chlorhexidine | Group 1: 6% triphalaGroup 3: Passive control group | On 1st day, after 48 hours and at 7th day | After 48 hGroup 1: 17%Group 2: 16%Group 3: -6%At 7 daysGroup 1: 44%Group 2: 45%Group 3: -2% | Group 1 and 2 differed significantly from control. No significant difference between group 1 and group 2. |
| 4 | Velmurugan A et al., 2013 [10] | Group 3: Chlorhexidine | Group 1: 20% aqueousextract of T. chebula Group 2: 20% aqueousextract of E. officinalis | Before and after 90 minutes | Group 1: 68%Group 2: 78%Group 3: 65% | Group 2 is significantly better than group 1 and group 3. |
| 5 | Khairnar MR et al., 2015 [33] | Group 1: Chlorhexidine | Group 2: Cranberry | On 1st day and at 14th day | Group 1: 69%Group 2: 68% | No statistically significant difference between group 1 and group2 |
| 6 | Yadav M et al., 2017 [34] | Group 2: Chlorhexidine | Group 1: 2% Green Coffee bean extractGroup 3: Sterile water | On 1st day and after 14 days | *Group 1: 51±32*Group 2: 55±27*Group 3: 7±16 | No statistically significant difference between group 1 and group 2. Group 1 and 2 differed significantly from control. |
| 7 | Usha C et al., 2017 [35] | Group 1: Chlorhexidine | Group 2: 0.5% Stevia mouthwash | On 1st day and at 8th day | Group 1: 100%Group 2: 100% | No statistically significant difference between group 1 and group2 |
*Mean values expressed in mean and standard deviation
Two studies established the effect of Triphala on Streptococcus mutans by comparing its antibacterial efficacy with chlorhexidine. In a study conducted by Srinagesh J et al., there was a significant reduction in the Colony Forming Units (CFU) of mutans streptococci in both Triphala (83% and 67%) and chlorhexidine (80% and 65%) at 15 and 45 days respectively in a sample of 57 people. In another study done by Srinagesh J et al., a CFU reduction of 17% and 44% was found at 48 hours and seven days respectively in Triphala group while a reduction of 16% and 45% was seen in chlorhexidine group in a total sample size of 60 subjects [Table/Fig-4].
Velmurugan A et al., have compared the antibacterial efficacy of Emblicaofficinalis, Terminaliachebula with chlorhexidine [10]. The results projected a marked decrease in streptococcus mutans in all the three groups i.e., 78.1% in E.officinalis group followed by 67.8% in T.Chebula group, and 65.0% in chlorhexidine group post 90 minutes of usage in a total sample size of 45 subjects [Table/Fig-4].
Usha C et al., compared the anticariogenicity of Stevia rebaudiana with chlorhexidine and found 100% Streptococcus mutans reduction in both the groups post eight days of administration in a total sample of 46 [35].
In a clinical trial by Khairnar MR et al., chlorhexidine was compared with cranberry and it was found that there were a 69% CFU reduction in chlorhexidine group and 68% CFU reduction in cranberry group after two weeks in sample of 50 patients [Table/Fig-4] [33].
Yadav M et al., compared green coffee bean extract with chlorhexidine and found 51.5 % streptococcus mutans reduction in coffee group and 55.60% reduction in chlorhexidine group after two weeks in a sample of 45 subjects [Table/Fig-4] [34].
Discussion
Mouthwashes are solutions or liquids used to rinse the mouth for a variety of purposes such as to remove or destroy bacteria, to act as an effective astringent, to deodorise the oral cavity and above all to exert a medicinal effect by relieving infection and to prevent dental caries [36].
According to Kornman KS, topical antimicrobials can be divided into two categories or generations based on their pharmacological properties, while the first generation can kill bacteria on contact (e.g., Cetylpyridinium chloride and sanguinarine), the second generation exerts a similar effect but with a more sustained antimicrobial action (e.g., Chlorhexidine) [37].
It is been proved time and again that mouthwash is a simple and acceptable method to deliver topical medicaments into the oral cavity for a vast majority of the population. In case of caries, the main therapeutic goal would be to reduce lesion progression or reverse the activity of the enduring ones [38].
In a systematic review done by Kumar S et al., it was reported that infrequent tooth brushing was linked to higher caries increments than frequent tooth brushing [39]. It has also been reported that other than the delivery of fluoride ions from the toothpaste, brushing frequently by itself has no additional benefit in preventing dental caries [40].
Mouthwashes in addition to mechanical plaque control measures, have been found to be very effective in reducing the oral microbial load [41]. Rinsing with a chlorhexidine mouthwash is arguably the most effective method to date [11]. The dental armory is incomplete without chlorhexidine as a mouth rinsing agent and has therefore, earned the eponym of the gold standard [42].
Chlorhexidine is an effective broad-spectrum antimicrobial agent which works on the principle of opposite charges attract. Chlorhexidine are positively charged (cations) that bind to negatively charged (anions) bacteria and surface structures in the oral cavity. Chlorhexidine exhibits its antimicrobial effect by binding to microbial cell walls, damaging the surface structure in the process eventually leading to an osmotic imbalance with consequent precipitation of cytoplasm causing cell death. The retention period of chlorhexidine in the oral cavity have been reported to be of up to 12 hours or longer depending on the dosage and form. Chlorhexidine is touted to be safe and also possesses an inherent advantage over antibiotics by not producing resistant microorganisms [43].
Loesche WJ in 1976 proposed the specific plaque hypothesis which emphasised that only a few species in the dental plaque biofilm are involved in caries, the main etiological agent being Mutans streptococci [44]. It has been hypothesised that patients with low Mutans streptococci population have low caries activity on the other hand, patients with high levels of Mutans streptococci have high caries activity [45]. Chlorhexidine is a very powerful bactericidal agent for Mutans streptococci, which is undoubtedly the most noteworthy group of bacterium associated with caries. However, chlorhexidine molecules attach to the surfaces of Mutans streptococci and initiate cell demise.
Since the 1970s, chlorhexidine have been used successfully in the dental profession for over four decades, its clinical efficacy and side effects pertaining to tooth staining and altered taste perception being well known to the profession. Staining of teeth can be attributed to the interaction of chlorhexidine molecules with chromogens present in food and beverages [46].
Natural products are secondary metabolites synthesised by an organism which behave as defense mechanisms against a vast majority of competing flora and fauna [47]. Throughout history, the inclusion of natural products in oral health has been investigated upon. The evidence for which was decoded from the Ebers Papyrus, highlighting a wide variety of recipes for mouthwashes made up of naturally occurring substances [48].
Extensive studies have been carried out on selected foods and beverages like tea, coffee, grape, propolis, shiitake mushrooms or traditional herbs. The presence of compounds exhibiting antibacterial activity against various pathogens, antiadhesive and inhibitory activity against the extracellular polysaccharide have been identified, demonstrated and confirmed using animal and human tests [49].
Some of the natural products have already been incorporated into mouthwashes and chewing gums with the sole aim of harnessing their beneficial medicinal properties [33,34].
Through various meticulous researches, it was brought into being that the polyphenolic compounds are the most potent of all compounds available in natural products.
Quality Assessment
The quality assessment of included trials was undertaken independently as a part of data extraction process. Four main quality criteria were examined.
Method of Randomisation, recorded as:
Yes-Adequate as described in the text
No-Inadequate as described in the text
Unclear in the text
Allocation Concealment, recorded as:
Yes-Adequate as described in the text
No-Inadequate as described in the text
Unclear in the text
Outcomes including assessors blinded to intervention, recorded as:
Yes-Adequate as described in the text
No-Inadequate as described in the text
Unclear in the text
Completeness of follow-up (was there a clear explanation for withdrawals and dropouts in each treatment group) assessed as:
Yes-Dropouts were explained
No-Dropouts were not explained
None-No Dropouts or withdrawals
Other methodological criteria examined included:
Presence or absence of sample size calculation
Comparability of groups at the start
Clear inclusion/ exclusion criteria
Presence/absence of estimate of measurement error. The validity and reproducibility of the method of assessment.
Risk of Bias in included studies:
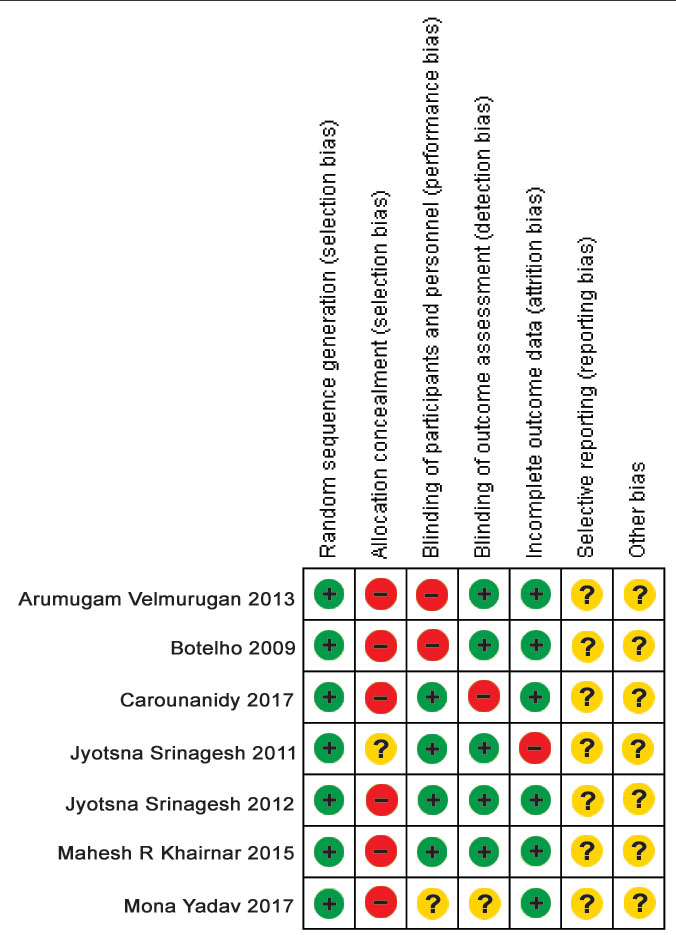
The assessments for the four main methodological quality items are shown in [Table/Fig-5] [4,10,31-35]. The study was considered to have a “High risk” of bias if it did not record a “Yes” in three or more of the four main categories, “Moderate” if two out of four categories did not record a “Yes”, and “Low” if randomisation assess or blinding and completeness of follow-up were considered adequate.
Risk of bias-major criteria [4,10,31-35].
| S.No | Study | Randomisation | Allocation Concealed | Assessor Blinding | Dropouts Described | Risk of Bias |
|---|
| 1 | Botelho MA et al., [31] | Yes | No | Yes | None | Low risk |
| 2 | Srinagesh J et al., [32] | Yes | Unclear | Yes | Yes | Low risk |
| 3 | Srinagesh J et al., [4] | Yes | No | Yes | None | Low risk |
| 4 | Velmurugan A et al, [10] | Yes | No | Yes | None | Low risk |
| 5 | Khairnar MR et al, [33] | Yes | No | Yes | None | Low risk |
| 6 | Yadav M et al., [34] | Yes | No | Unclear | None | Moderate risk |
| 7 | Usha C et al., [35] | Yes | No | No | None | Moderate risk |
Report on quality of evidence looked upon:
Seven trials were included in this review. All seven studies included in this review had a level of evidence 2 [Table/Fig-6]. Thus, the level of evidence is high.
Evidence level of selected articles [4,10,31-35].
| S. No | Author | Year | Study Design | Level of Evidence |
|---|
| 1 | Botelho MA et al., [31] | 2009 | Randomised clinical trial | Level 2 |
| 2 | Srinagesh J et al., [32] | 2011 | Randomised clinical trial | Level 2 |
| 3 | Srinagesh J et al., [4] | 2012 | Randomised clinical trial | Level 2 |
| 4 | Velmurugan A et al., [10] | 2013 | Randomised clinical trial | Level 2 |
| 5 | Khairnar MR et al., [33] | 2015 | Randomised clinical trial | Level 2 |
| 6 | Yadav M et al., [34] | 2017 | Randomised clinical trial | Level 2 |
| 7 | Usha C et al., [35] | 2017 | Randomised clinical trial | Level 2 |
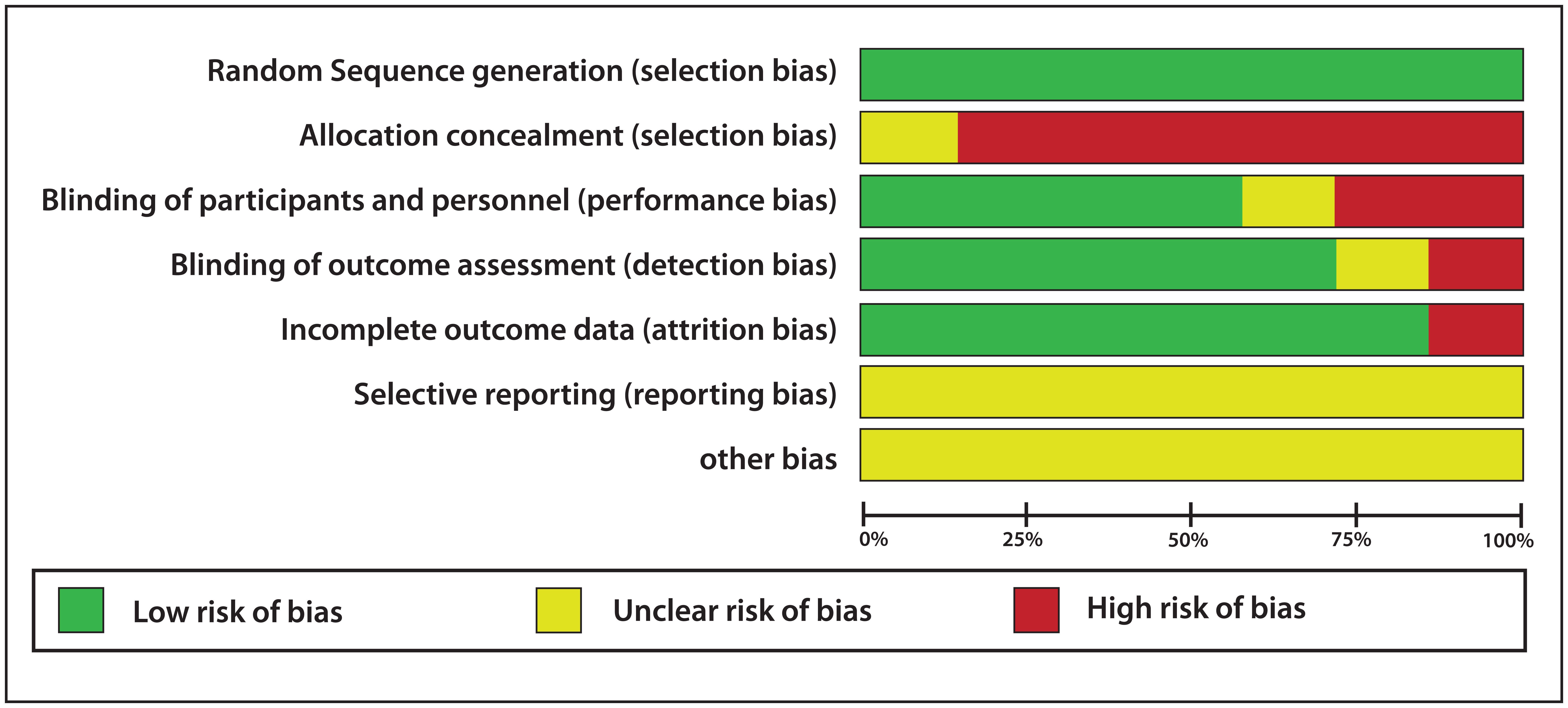
The risk of bias for all the studies included in the present systematic review was assessed using Cochrane criteria [Table/Fig-5,7], the summary and graph were generated using Review Manager 5 software (RevMan 5) [Table/Fig-8,9]. Four parameters were evaluated to assess the risk of bias on individual studies. Five out of seven studies showed low risk of bias and the remaining two showed moderate risk of bias. Moderate risk of bias was shown in the study conducted by Yadav M et al., as allocation concealment was not done and there was no clear evidence of assessor blinding [34]. Moderate risk of bias was seen in the study conducted by Usha C et al., as allocation concealment and assessor blinding was not done [35]. Low risk of bias was shown in the study conducted by Srinagesh J et al., as randomisation, assessor blinding and dropouts or withdrawals if any, were well explained [32]. Randomisation and assessor blinding were done and well described in the trials reported by Botelho MA et al., Srinagesh J et al., Velmurugan A et al., and Khairnar MR et al., also, there were no incidence of dropouts or withdrawals in the aforesaid clinical trials as well [4,10,31,33]. Therefore, the five studies discussed above have low risk of bias.
Risk of bias-minor criteria [4,10,31-35].
| S. No | Study | Sample Justified | BaselineComparison | I/E Criteria | Method Error |
|---|
| 1 | Botelho MA et al, 2009 [31] | Yes | Yes | Yes | No |
| 2 | Srinagesh J et al, 2011 [32] | Yes | Yes | Yes | No |
| 3 | Srinagesh J et al, 2012 [4] | No | Yes | Yes | No |
| 4 | Velmurugan A et al., [10] | No | Yes | Yes | No |
| 5 | Khairnar MR et al., [33] | Yes | Yes | Yes | No |
| 6 | Yadav M et al., [34] | Yes | Yes | Yes | No |
| 7 | Usha C et al., [35] | No | Yes | Yes | No |
Srinagesh J et al., described the limitations of their study emphasising that only short term effect of triphala was assessed against oral streptococci [32]. Therefore, more trials are required to explore long term antibacterial efficacy of triphala against various oral microorganisms. Yadav M et al., explained the limitations pertaining to small sample size and that more samples have to be incorporated in order to further validate the results [34].
From this systematic review it can be concluded that natural products mouthwash can be used as an alternative to chlorhexidine as both showed similar antibacterial efficacy against Streptococcus mutans.
Natural products containing mouthwash can ideally be used as an alternative to chlorhexidine as both have shown similar antibacterial efficacy.
In future, research should be aimed at conducting prospective studies to validate the results.
Report of Outlier Data
No outlier data obtained.
Conclusion
The present systematic review does not provide concrete evidence to show increased antibacterial efficacy of natural products as compared to chlorhexidine. Five articles included in this review have a low of risk bias whereas two articles have shown a moderate risk of bias. The aforesaid articles also have a less follow-up period. Thus, the present systematic review recommends to have a long term follow-up of the sample size. It also recommends more studies to be done comparing other natural products as well other than those incorporated in the aforesaid articles with chlorhexidine coupled with more standardised techniques other than CFU to have a more reliable outcome.