Fine Needle Aspiration Cytology (FNAC) is a quick, sensitive and inexpensive method to diagnose benign and malignant palpable lesion [1]. Its utility has amplified in the diagnosis of deep organ lesions when combined with image guidance techniques such as conventional ultrasound, computerized tomography [2,3] or transendoscopic ultrasound [4-6]. Sonography and Computed Tomography (CT) both provide adequate image guidance for FNAC and both have their benefits and disadvantages. FNAC under sonographic guidance has advantage over CT guidance are portability, shorter procedure time, lack of ionizing radiation, real time image visualization of needle image and lower test cost; which makes sonographic guided FNAC technique preferable for most location of the lesion in the body [7-11]. The ability to see through bowel gas and bone as well as thick soft tissue, superior delineation of the lesion and adjacent structures, broad field of view, and relative ease of needle visualizaton are some of the reasons why CT is preferred in many institutions for providing guidance for FNAC of lymph nodes in abdomen and retroperitoneum [12-18].
The aim of present study was to evaluate adequacy of material, cytologic assessment of samples obtained by image-guided aspiration and side effect/complication.
Materials and Methods
This descriptive retrospective study was performed in the Department of Pathology and Radiology, SN Medical college, Agra. Advanced test were outsourced to Lal pathlabs, Kanpur, Uttar Pradesh, India. The data were obtained by reviewing the laboratory database and cytology reports of the FNACs performed between September 2009 and October 2011 and 60 cases were included. The aspirated lymph nodes were located in Mesenteric (n=20), para-aortic (n=24), pelvic (n=2), peri-pancreatic (n=10) and porta-hepatis (n=4). The diameter of node ranged from 1 to 5 cm (mean 2.5 cm). A total of 56 lesions were non-palpable from outside. Four lesions were palpable, however presented as diffusely ill-defined swelling. Written informed consent and clinical details were obtained from each patient. Test results of HIV, HCV and HbsAg were recorded and due precaution were taken on aspiration. The complete coagulation profiles were obtained from each patient before FNAC. Imaging report was cross checked at the time of guided FNAC and the feasibility of procedure was determined. Gray scale imaging was used for localization and shortest possible route with no major vessels was selected. Except for porta hepatis lymph nodes aspiration (transhepatic), route through solid organs was avoided. The site of FNAC was cleaned by using spirit and betadine with no local anaesthesia; however intravenous sedation and local anaesthesia were used in paediatrics and over anxious patient respectively. The sonographic machine used was RT 3600 (Japan) with sterile 3.5 or 5 MHz micro convex sector probe for guidance was used. The CT unit used was Somatom Hi Q (Siemens). On visualization of the lesion, 9.0 cm length (22 guage) needles with a stylet using the free hand technique was advanced into the lesion under continous real-time US guidance. When needle tip was in lesion stylet was removed, suction was applied using a 20 mL syringe by pulling piston from handle and patients were asked to hold or take slow breath only till aspirated material was obtained. Minimum of two passes were taken for each patient.
Aspirated material was spread and smeared over minimum of 3-4 glass slides. Smears were kept for air dried or fixed in alcohol and stained with May-Grunwald-Giemsa (MGG) or Haematoxylin and Eosin (H&E) respectively. Special stains like Ziehl Neelson stain for Acid Fast Bacilli (AFB) and Periodic Acid Schiff (PAS) for fungus were done whenever required. Cell block preparation was made whenever lesion appeared doubtful for malignancy. Immunohistochemistry was performed on cell block to reach diagnosis in cases of difficult interpretation. Each patient was monitered for at least one hour after aspiration to check procedure related immediate complications and was asked to come after 48-72 hours for acute and after seven days for late complications. The smears were interpreted by two observers Without bias, study was further validated by six monthly follow-up periods of five years due partial lack to confirm the cytological diagnosis. Confirmation of cytological diagnosis was correlated and confirmed by clinical findings, imaging, histopathological diagnosis, follow-up and status of remission after treatment.
Results
There were total of 60 cases aspirated in which, sonographic guided FNAC was done in 56 patients and CT guided FNAC in four patients. Adequate material for cytologic diagnosis was obtained in 58(96.6%) patients with a similar diagnostic accuracy and two (3.4%) patients showed blood only. Most common group of lymph nodes involved was para-aortic (n=24) then mesenteric (n=20) [Table/Fig-1]. M:F ratio was 2.16:1 and age range was 8 to 83 years. On distribution of cases according to age groups there were bimodal increase incidence first in 21-30 (n=12; 20%) and second in 51-60 (n=12; 20%) followed by 61-70 (n=11; 18.3%). Incidence of tuberculosis was more common in female than male however metastatic carcinomas and lymphomas were commoner in males.
Distribution according to site.
| S. No. | Site | No. of Cases | % |
|---|
| 1. | Mesenteric lymph nodes | 20 | 33.3% |
| 2. | Para-aortic lymph nodes | 24 | 40% |
| 3. | Pelvic lymph nodes | 2 | 3.3% |
| 4. | Peri-pancreatic lymph nodes | 10 | 16.7% |
| 5. | Porta-hepatis lymph nodes | 4 | 6.7% |
| Total | 60 | 100% |
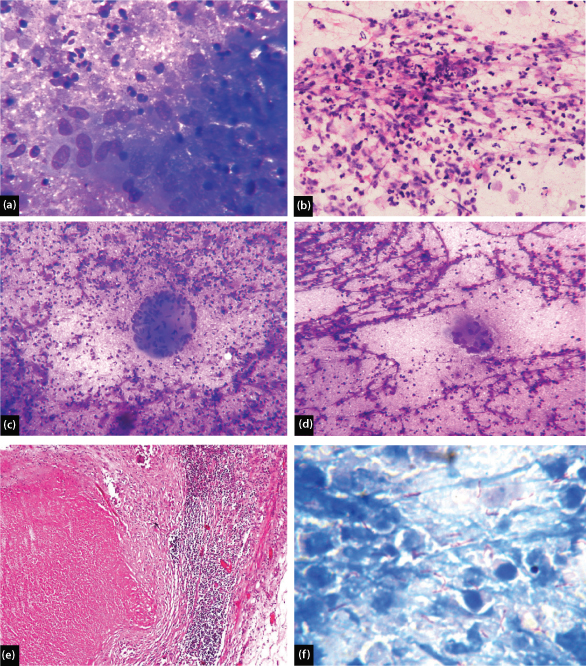
As [Table/Fig-2] shows final cytological report of all patients. Out of 60 cases there were 16 (26.7%) cases which show caseous necrosis, thin pus and cellular material having epithelioid cells, lymphocytes, macrophages, giant cells and granulomas indicative of tubercular etiology [Table/Fig-3a-e]. Ziehl-Neelsen stain was performed which showed AFB in 12 (75%) patients, in which one patient showed numerous AFB [Table/Fig-3f]. A 100% AFB positivity is seen in patients having necrosed background with or without cellular matter. No AFB positivity is seen in patients having granulomas only (n=3) which were later confirmed by histopathology and TB-PCR [Table/Fig-4]. All of these patients improved after treatment with antitubercular drugs and showed positive clinical and radiological outcome.
Distribution according to cytological diagnosis.
| S. No. | Types of Lesion | Lesion | No. of Cases | % |
|---|
| 1. | Reactive | Tubercular lymphadenitis | 16 | 26.7% |
| 2. | Benign | - | 0 | 0.0% |
| 3. | Malignant | Hodgkin’s lymphoma | 3 | 5.0% |
| Non-Hodgkins lymphoma | 17 | 28.3% |
| Metastatic adenocarcinoma | 13 | 21.7% |
| Metastatic squamous cell carcinoma | 1 | 1.7% |
| Metastatic Adeno-squamous carcinoma | 2 | 3.3% |
| Metastatic Seminoma | 3 | 5.0% |
| Metastatic Non-seminomatous germ cell tumour | 2 | 3.3% |
| Metastatic neuro-endocrine tumour | 1 | 1.7% |
| 4. | Inadequate | - | 2 | 3.3% |
| Total | 60 | 100% |
(a) Epithelioid Granuloma in purulent background (MGG, 400X); (b) Tuberculous lymph node showing cellular material showing predominantly inflammatory cells (H&E, 400X); (c) Tuberculous lymph node showing Langhan’s giant cell (MGG, 100X); (d) Tuberculous lymph node showing Langhan’s giant cell with cellular material in necrotizing background (MGG, 100X); (e) Tuberculous lymph node showing Langhan’s giant cell with central necrosis (H&E 100X); (f) Numerous acid fast bacilli in smear (Z.N. Stain, 1000 X).
Tuberculosis (n-16) cases with AFB status and further confirmation.
| Types | No. of Cases | Confirmed by |
|---|
| Biopsy | RT-PCR |
|---|
| AFB+ | 12 | 0 | 0 |
| AFB- | 4 | 3 | 1 |
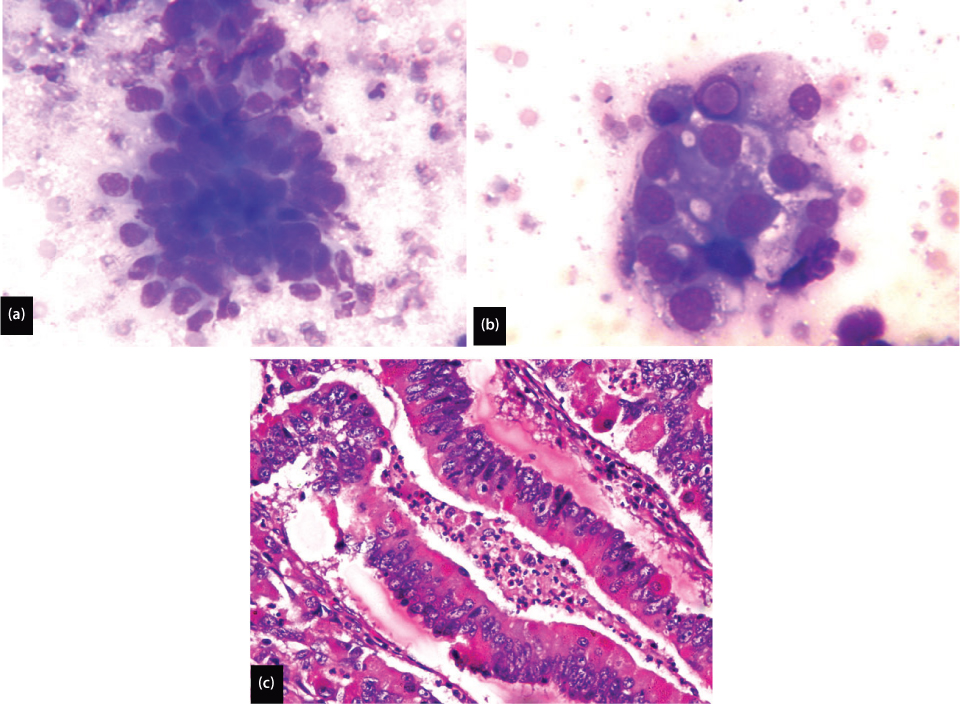
Of 22 metastatic lesions encountered, 13 were adenocarcinoma [Table/Fig-5a] in which six cases revealed intracellular mucin. One case revealed intranuclear inclusion [Table/Fig-5b]. One case of enlarged peri-pancreatic and retroperitoneal lymph nodes showed predominantly loosely cohesive clusters of small round cell with focal suspicion of acini formation. Fair number of mitotic figures and atypical mitoses were also noted [Table/Fig-5c]. All of these features favour the diagnosis of metastatic adenocarcinoma over non-Hodgkin’s lymphoma. From remaining nine cases of metastatic carcinoma, one was metastatic squamous cell carcinoma, two metastatic adeno-squamous carcinoma, three metastatic seminoma, two metastatic non-seminomatous germ cell tumour and one metastatic neuro-endocrine tumour. Metastatic non seminomatous germ cell tumour showed a combination of various components these include, embryonal carcinoma forming predominant component, while yolk sac and teratoma were minor component.
(a) Metastatic adenocarcinoma showing well formed acini (MGG, 400X); (b) Metastatic adenocarcinoma showing intranuclear inclusion (MGG, 1000X); (c) Metastatic adenocarcinoma showing abnormal glands with nuclear pseudo-stratification, coarse chromatin, prominent nucleoli and atypical mitosis (H&E, 400X).
After five years of follow-up, of 22 metastatic lesions, 15 patients had probable known primary site of malignancy and seven patients were advised to look for primary. In these seven patients of unknown primary, clinico-radiological correlation and immunohistochemistry was performed and probable primary lesion was informed. These are:
One metastatic neuro-endocrine carcinoma – Primary neuro-endocrine tumour from jejunum;
One metastatic squamous cell and two adeno-squamous cell carcinoma – Primary from cervix;
Three metastatic adenocarcinoma – two had primary in pancreas and one had primary in ampulla of Vater in duodenum.
Out of remaining 15 cases of metastatic carcinoma, four were lost on follow-up. From 11 cases, eight cases had known primary lesion confirmed by cytological diagnosis from primary tumour on same sitting. Resection biopsy of intestine was done in three cases of metastatic adenocarcinoma and was confirmed on histopathological examination.
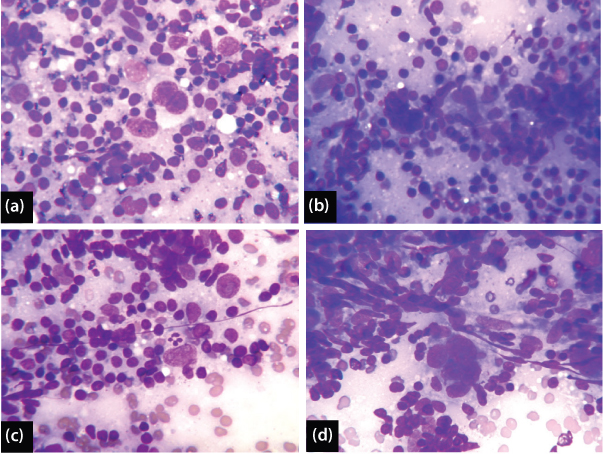
There were three patients diagnosed cytologically as Hodgkin’s lymphoma. Two cases showed classical Reed-Sternberg cell [Table/Fig-6a], however few mononuclear variant known as Hodgkin’s cell, multilobated, multinucleated and occasional embryoid cells were also encountered in all the cases [Table/Fig-6b-d]. Two cases did not show eosinophils even after deliberate search. Well formed granulomas were observed in two cases, whereas scattered histiocytes were seen in all the three cases. There was abundance of blood vessels and lymphoid tangles in all the three cases. Two cases showed fair number. of follicular dendritic cells. Three cases of Hodgkin’s lymphoma, all confirmed by histopathological examination in which two were nodular sclerosis and one was mixed cellularity. A total of 17 patients were of non-Hodgkin lymphoma of which eight were large cell [Table/Fig-7a], two lymphoplasmacytic, four small lymphocytic, one follicular centre cell (predominantly centrocytic) and two anaplastic large cell lymphoma according to WHO classification.
(a) Hodgkin’s lymphoma showing characteristic Reed – Sternberg cell (MGG, 400X); (b) Multi-lobated Reed- Sternberg cell in Hodgkin’s Lymphoma (MGG, 400X); (c) Abundant Mono-nuclear cells in Hodgkin’s Lymphoma (MGG, 400X); (d) Multi-nucleated Reed-Sternberg cell in Hodgkin’s Lymphoma (MGG, 400X).
(a) Scattered population of atypical lymphoid cells in non-Hodgkin’s lymphoma (H&E, 1000X); (b) Lymphoplasmacytic lymphoma showing intracytoplasmic Russell bodies (H&E, 400X).
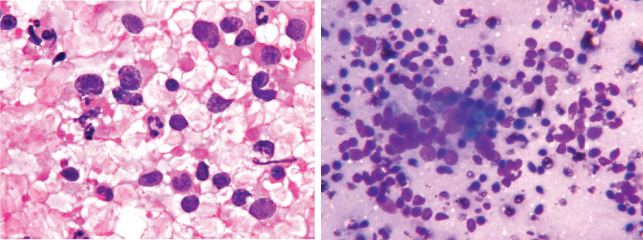
Two cases of non-Hodgkin’s lymphoma showed mixture of mature lymphocytes, plasma cells, lymphocytes with plasmacytoid features and plasmacytoid immunoblast and few bi and multinucleated plasma cells. Occasional Russell bodies were also noted [Table/Fig-7b]. This led to some overlap between lymphoplasmacytic lymphoma and plasmacytoma. Two cases non-Hodgkin’s lymphoma revealed large bizarre cells with horse-shoe or embryoid shaped nucleus (R-S like cells). Phagocytosis was evident in few cells. Large number of R-S like cells and lack of reactive background population were against Hodgkin’s lymphoma. Some overlap with anaplastic neoplasms were thus noted.
Histopathology was done in two cases which reported small lymphocytic lymphoma. Seven cases were confirmed from different super speciality centres by histopathology and are having symptomatic remission after chemotherapy.
A total of 17 cases of Non-Hodgkin’s lymphoma after five year follow-up, five cases were lost on follow-up and three cases died after voluntarily refusal for further management [Table/Fig-8].
Confirmation of cytological diagnosis of the lesions.
| Lesion on cytological diagnosis | No. of cases | Confirmed diagnosis by histology or follow-up (repeat FNAC by others (2nd) | Lost in follow-up |
|---|
| Hodgkin’s lymphoma | 3 | 3 | 0 |
| Non-Hodgkin’s lymphoma | 17 | 4 Small Lymphocytic Lymphoma (SLL) (2 FNAC 2nd and 2 histology)6 large cell, high grade lymphoma (?DLBCL) (FNAC)2 DLBCL (by histology)2 Lympho-plasmacytic lymphoma (FNAC)1 Follicular lymphoma (2nd FNAC)2 Anaplastic Large Cell Lymphoma (ALCL) (1 FNAC 2nd and 1 histology) | 5 (Lost after 1 year follow-up) |
| Metastatic Adenocarcinoma | 13 | - 2 Mucinous adenocarcinoma of ovary- 3 Carcinoma colon- 2 Carcinoma pancreas- 1 carcinoma ampulla of vater in duodenum- 1 Gastric carcinoma | 4 |
| Metastatic squamous cell carcinoma | 1 | Carcinoma cervix | 0 |
| Metastatic Adeno-squamous carcinoma | 2 | Carcinoma cervix | 0 |
| Metastatic Seminoma | 3 | Testicular seminoma | 0 |
| Metastatic Non-seminomatous germ cell tumour | 2 | Embryonal carcinoma with minor yolk sac tumour component | 0 |
| Metastatic neuro-endocrine tumour | 1 | Neuro-endocrine tumour of jejunum | 0 |
Inadequate material for diagnosis was obtained from two patients had small lymph node (<1cm). One was from porta hepatis lymph node and one was from para-aortic lymph node.
Procedure was well tolerated by all patients. All patients were observed for minimum of one hour. Local pain was the most common immediate symptom which subsided after taking analgesics. There was no acute or late complication encountered in any of the patient.
Discussion
FNAC is a quick, sensitive and inexpensive technique for diagnosing benign and malignant palpable lesions [1] and non-palpable lesions from which material can be obtained with image-guidance [19]. The greatest advantage of this minimally invasive technique is its high sensitivity and specificity [20]. Imaging modalities such as CT and US have been widely used for FNAC of deep seated lymph nodes [4-6]. The advantage of using sonographic rather than CT guidance include lack of ionizating radiation, portability, shorter procedure time, real time visualization of needle and lower cost [8,21,22] but, the ability to see through bowel gas and bone as well as thick soft tissue, superior delineation of the lesion and adjacent structures, broad field of view, and relative ease of needle visualizaton are some of the reasons why CT is preferred in many institutions for providing guidance for FNAC of lymph nodes in abdomen and retroperitoneum [13-18].
A total of 60 cases were aspirated and specific diagnosis was rendered in 58 cases. Two cases contained only blood, thus were inadequate.
The adequacy of lymph node aspiration under image guidance vary from 85% to 97.2%. Our study showed adequacy of 96.7% which is comparable to study of Tikkakoski T et al., [9]. The adequacy of other studies were lower than the present study [Table/Fig-9] [9,21-23].
Summary of adequacy of intra-abdominal lymph nodes FNAC in patients reported in series [9,21-23].
| S. No. | Study | Adequacy |
|---|
| 1. | Tikkakoski T et al., [9] | 97.2% |
| 2. | Suri R et al., [23] | 85.2% |
| 3. | Memel DS et al., [21] | 91.0% |
| 4. | Fisher AJ et al., [22] | 86.0% |
| 5. | Present study | 96.7% |
Males were more frequently subjected to FNAC as compared to females, but in studies of Tikkakoski T et al., and Memel DS et al., the difference was in reverse direction [9,21]. Present study showed Male:Female ratio of 2.6:1 which is fairly close to the study of Al-Mofleh IA et al., [11]. This signifies that intra-abdominal tumours are less common in females as compared to males. The common malignancies in female originate from genital tract in which most common is carcinoma cervix and these tumour are generally symptomatic earlier in contrast to intra-abdominal lesions which manifest generally late.
The most common site of the aspiration in present study were para-aortic and mesenteric lymph nodes but peri-pancreatic lymph nodes were also involved in significant numbers.
In the current study of 60 cases, specific diagnosis was evaluated in 58 cases, of which 16 (27%) were non-malignant (infectious) and 42 (73%) were malignant [Table/Fig-10]. The percentage of malignant and non malignant cases were fairly similar to that observed by Memel DS et al., [21] and lie in between the studies of Al-Mofleh IA et al., and Fisher AJ et al., [11,22].
Summary of malignant and non–malignant lesions reported in series [11,21-23].
| S. No. | References | No. of cases | Specific diagnosis rendered in cases | Malignant | Non-malignant |
|---|
| 1 | Fisher AJ et al., [22] | 35 | 30 | 26 (86.7%) | 4 (13.3%) |
| 2 | Al-Mofleh IA et al., [11] | 37 | 33 | 17 (51.5%) | 16 (48.5%) |
| 3 | Suri R et al., [23] | 102 | 87 | 47 (54.0%) | 40 (46.0%) |
| 4 | Memel DS et al., [21] | 23 | 21 | 16 (76.2%) | 5 (23.8%) |
| 5 | Present study | 60 | 58 | 42 (72.4%) | 16 (27.6%) |
Tuberculosis was diagnosed in all the 16 patients of benign lesion either conclusive or suggestive, depending upon AFB positivity. Frequency of tuberculous lymph nodes (conclusive/suggestive) in our study was fairly comparable with Suri R et al., [23]. Other studies reported a wide variation in frequency ranging from 3% (Fisher AJ et al.,) [22] to 45.5% (Al-Mofleh IA et al.,) [11]. Granulomas (with or without lymphocytes) were noted in seven cases. Giant cells were seen in two cases. Singly scattered epithelioid cells were seen in a total of seven cases (7/16) of these six were associated with granulomas and one without granuloma. Among six which were associated with granulomas, two had lymphoid background, three had purulent background and one had caseous background. The one, which was not associated with granuloma had purulent background. AFB was sought in aspirates after Ziehl Neelsen Staining. Total of 12 (75%) cases out of 16 were positive. The maximum positivity was seen in smear which showed caseous necrosis only (100%) and minimum in the cases with non-necrotizing granulomas (0%). No fungal profile was seen in any case. All patients showed radiological and clinical improvement after taking anti-tubercular therapy.
Non-Hodgkin’s lymphoma was the most common primary nodal malignancy seen in 17(40.4%) patients similarly observed in the studies of Al-Mofleh IA et al., and Suri R et al., [11,23]. Problematic areas on morphology are sub-categorization of cases of large cell lymphoma on cytology, differentiation of paraimmunoblastic variant of B-SLL/CLL and classical B-SLL/CLL with proliferation centres and cytological differentiation between follicular lymphoma, predominantly centrocytic type and mantle cell lymphoma. In these cases histopathological confirmation is required.
Hodgkin’s Lymphoma was diagnosed in three patients, eosinophils are fairly characteristic of Hodgkin’s Lymphoma but two of the three cases virtually devoid of eosinophils was subjected to histology and the diagnosis was confirmed. Expression of concomitant fibrosis presents as Nuclear crushes and lymphoid tangles. Another thing that was noticed was the presence of well formed granulomas in two out of the three cases. A study by Koo V et al., showed that out of cases diagnosed as granulomatous on cytology, 22% turned out to be Hodgkin’s lymphoma on histology [24]. Rashmi Kumari TR et al., were helpful which pointed out the presence of well formed granulomas in an appropriate background to be a useful indicator for Hodgkin’s Lymphoma [25].
Metastases is the most common diagnosis in our study given in 22 (36.7%) patients which included 13 adenocarcinomas, one squamous cell carcinoma, two adeno-squamous carcinomas, three metastatic seminomas, two metastatic nonseminomatous germ cell tumours and one neuroendocrine tumour [11,21-23]. Fifteen patients had known primary tumour and seven patients were advised to look for primary (unknown primary). Indication of primary site was given after clinico-pathological correlation and immunohistochemistry on cell block preparation.
From recent study of Sumana BS et al., which showed diagnostic yield of USG guided FNAC of Intra-abdominal lesions was 96.77% with 3.23% of unsatisfactory smears [26]. Majority of cases were malignant (67.74%), which is very close to this study and validate that intra-abdominal tumours tend to be palpable or symptomatic late in due course of disease.
Procedure was well tolerated by all the patients. There was no acute or late complication encountered in any patient. Inadequate material for diagnosis was obtained from two patients who had small lymph node (<1cm). One was from porta hepatis lymph node and other was from para-aortic lymph node. Livraghi T et al., [27] reported a mortality rate of 0.008% with a 0.5% serious and a 0.49% minor complication rate. Nolsoe C et al., reported 0.2% morbidity with a mortality rate of 0.028% in a study of 3500 FNAC procedures [28].
After five year period of follow-up, total of three patients of Non-Hodgkin’s lymphoma, two diffuse large B-cell lymphomas and one anaplastic large cell lymphoma were died among primary malignant lesion. From 10 patients of metastatic carcinoma, nine from metastatic adenocarcinoma and one from metastatic adeno-squamous carcinoma were died. No mortality was noted from cases of tuberculosis and Hodgkin’s lymphoma [Table/Fig-11].
Final outcome of the lesions after 5-years of follow-up.
| S. No. | Lesion | Total no of cases | Remission | Lost on follow-up/death | Under treatment/not in complete remission |
|---|
| 1 | Tuberculosis | 16 | 16 | 0 | 0 |
| 2 | Hodgkin’s lymphoma | 3 | 3 | 0 | 0 |
| 3 | Non-Hodgkin’s lymphoma | 17 | 4 | 8 | 5 |
| 4 | Metastatic carcinoma | 22 | 5 | 14 | 3 |
Our study also showed that diagnosis made on FNAC is well correlated and confirmed by histopathological and radiological findings, which further validated the usefulness of FNAC procedure.
Limitation
1. Histopathological confirmation was not available in all cases of this study especially cases of non-Hodgkin’s lymphoma.
2. Sample size was relatively less.
3. Very long period of follow-up, which lend this research to miss recent data.
4. Patients lost on follow-up, further limit the validation of study.
Conclusion
FNAC of intra-abdominal lymph nodes through imaging guidance is a useful, cost-effective, safe diagnostic procedure and has a pivotal role for establishing tissue diagnosis as a primary investigative modality. It is also helpful and accurate in follow-up of patients with a known malignant disease, also enables diagnosis with fair degree of ease without resorting to major surgery merely for diagnostic purposes.
We strongly recommend that FNAC may be used as an alternative to conventional biopsy in the diagnosis of clinically suspected and unsuspected lymph node malignancy and to assess the stage of tumour.