Stroke is considered to be one of the major causes of morbidity and mortality globally and is associated with numerous medical complications leading to extended hospital stay and significant health care expenses [1]. Dysphagia is one of the most common and life-threatening conditions seen among the Post-stroke individuals [2]. Nearly 65% of stroke survivors exhibit some degree of impairment in their swallowing ability. Dysphagia and stroke are thoroughly associated, and aspiration pneumonia is considered to be one of the main cause of mortality in individuals with stroke [3,4]. These complications and psychosocial sequelae of dysphagia impose prompt and precise identification of individuals with dysphagia who are at risk, in order to enhance safe oral feed, pulmonary health, and improved quality of life.
Cough has been an area of interest in the evaluation and treatment of dysphagia [5]. Stroke is reported to have an adverse effect on cough function [6]. This finding is evident as the neural substrates and anatomical substrates are shared in common for respiration, swallowing and cough function.
Evidence suggests that cough airflow measures may serve as an effective physiologic metric to indicate airway defence capabilities in individuals who are at risk [6,7]. Current investigations also emphasise strong association between voluntary and reflexive cough airflow measures and airway maintenance during swallowing [8] investigated the voluntary and reflexive cough among the healthy individuals and individuals with ischaemic hemispheric stroke. They found that the peak cough flow rate and cough volume acceleration of voluntary cough and reflexive cough were impaired in individuals with stroke as compared to healthy individuals.
However to date, there has been no study attempted to investigate the cough acoustically among the neurogenic stroke populations. The aim of the study was to investigate the analysis of cough acoustically in individuals with dysphagia.
Materials and Methods
The study was conducted in the Department of Neurology, from September 2017 to March 2018. A cross-sectional research design with the convenient sampling was adopted. The protocol was approved by the Institutional Research committee and Institutional Ethics committee at Kasturba Hospital, Manipal (Ref no: 518/2017). The purpose and the procedure of the study were explained and a written informed consent was obtained from all the participants prior enrollment in the study. A total of 30 participants Post-stroke in the age range of 40-70 years (Mean±SD = 58.369.±49 years) were recruited based on the selection criteria [Table/Fig-1].
Framework of acoustic analysis using MFCC extraction and universal background model- Gaussian mixture model.
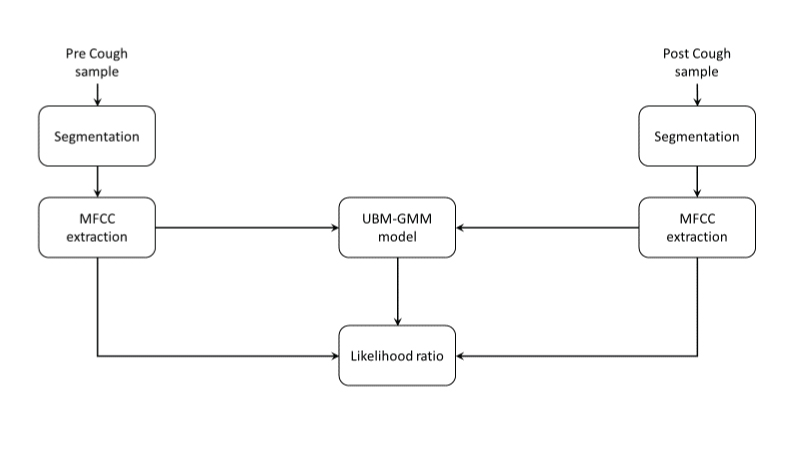
Inclusion Criteria
Individuals with acute post-stroke, adults in the age range of 41-70 years (Erikson’s stages of psychosocial development, 1968). Individuals who are on tube dependent for feed (FOIS – Level 2).
Exclusion Criteria
Tracheostomy/Ventilator/GCS <8. Neurodegenerative disorders, gastrointestinal disorders. Unable to follow instructions (Aphasia/cognitive problems/hearing loss/other associated conditions).
Instrumentation
Manipal Manual for Swallowing Assessment
Functional Oral Intake Scale
Laptop with PRAAT Software
AHUJA UTP-30 Microphone
Procedure
All the participants received stroke specific evaluations by the neurologist and dysphagia specific evaluation by the speech pathologist. The Clinical Swallowing Examination (CSE) was initiated, only for participants who adhered to the selection criteria of the study. The CSE was conducted using Manipal Manual for Swallowing Assessment [9]. The MMSA comprised of assessment of structure, function (sensory, motor) and phases of swallowing. The functional oral intake scale was administered [10]. It is a 7-point rating scale from no oral intake to total oral intake. The participants were selected only if they were rated as Level 2 for further evaluation.
The participants were made to sit comfortably in upright posture. The participants were instructed to cough voluntarily pre and post-swallow of water with a trial demonstration by the clinician. The participants were asked to cough on a trial basis to confirm if the instructions were comprehended by the participant. Once the clinician was confirmed on the same, the cough sample was recorded pre and post-swallow of 2 mL of water for each of the participants.
The cough stimuli were recorded using an AHUJA UTP-30 microphone at a distance of 8 cm from the participant’s mouth. The microphone was connected to the laptop installed with PRAAT software. The stimuli were recorded for three trails with gap duration of 10 seconds between each of the trails. Among the three trials, the best cough stimuli as determined by the clinician were subjected to perceptual and acoustic analysis.
Data Analysis
The cough stimuli were analysed for both perceptual and acoustical analysis pre and post-swallow for each of the participants.
Perceptual analysis of cough: The cough stimuli were perceptually analysed using 3-point rating scale by two experienced speech- language pathologists (clinical experience >10 years). It was rated as 0- very weak, 1-weak and 2-strong.
Acoustic Analysis of Cough
The cough samples were analysed pre and post-swallow conditions based on the Mel-Frequency Cepstral Coefficients (MFCC) with the sampling frequency 8 KHz; MFCCs 13 and window length of 20 sec.
Statistical Analysis
The statistical analysis was completed using SPSS 16.0. Descriptive Statistics was used for both perceptual and objective analysis. Cohen’s Kappa (k) was used as a measure of agreement between the raters to compare between pre and post cough samples perceptually. Wilcoxon signed rank test was used to compare between the pre and post-cough samples acoustically. A p-value less than 0.05 was considered statistical significant.
Results
A total of 30 participants were recruited out of which only 26 (19 males and 7 females) cough samples could be analysed (four samples were analysed due to reduced amplitude of the signal) in the age range of 40-70 years (Mean±SD = 58.369.±49 years) [Table/Fig-2].
FOIS Scores of the participants.
| FOIS Scores (Level 1–5) | Number of Participants (n=26) |
|---|
| Level 1 | Nil |
| Level 2 | 14 |
| Level 3 | 9 |
| Level 4 | 1 |
| Level 5 | 2 |
| Level 6 | Nil |
| Level 7 | Nil |
Tube dependent (levels 1-3); 1-No oral intake, 2 -Tube dependent with minimal/inconsistent oral intake, 3 -Tube supplements with consistent oral intake
Total oral intake (levels 4-7); 4 - Total oral intake of a single consistency, 5- Total oral intake of multiple consistencies requiring special preparation, 6- Total oral intake with no special preparation, but must avoid specific foods or liquid items, 7 Total oral intake with no restrictions
On MMSA, structurally, it was observed that majority of the participants exhibited deviations of lips. Functionally, most of the participants were pooling in the range of mild to moderate impairment, predominantly on the moderate category (based on scoring as per MMSA manual). On phases of swallowing, (thin liquid consistency; water), it was observed that most of the participants were observed to be in the group of mild to moderate impairment, predominantly with moderate impairment (based on scoring as per MMSA manual). The other findings observed were absence of gag reflex, acute pain during swallowing, poor oral hygiene, dehydration, loss of weight, delayed oral transit, reduced laryngeal excursion during the feed trial [Table/Fig-3].
MMSA Scores of the participants.
| Participants(n=26) | MMSA(Manipal Manual of Swallowing Ability) |
|---|
| Sensory | Motor | Phases of Swallowing |
|---|
| 1 | 0 | 1 | 1 |
| 2 | 1 | 1 | 1 |
| 3 | 0 | 1 | 1 |
| 4 | 1 | 1 | 1 |
| 5 | 0 | 1 | 1 |
| 6 | 1 | 1 | 1 |
| 7 | 1 | 1 | 1 |
| 8 | 0 | 1 | 1 |
| 9 | 1 | 1 | 1 |
| 10 | 0 | 1 | 1 |
| 11 | 1 | 1 | 1 |
| 12 | 0 | 1 | 1 |
| 13 | 1 | 1 | 1 |
| 14 | 0 | 1 | 1 |
| 15 | 0 | 1 | 1 |
| 16 | 1 | 1 | 1 |
| 17 | 0 | 1 | 1 |
| 18 | 1 | 1 | 1 |
| 19 | 1 | 1 | 1 |
| 20 | 0 | 1 | 1 |
| 21 | 0 | 1 | 1 |
| 22 | 1 | 1 | 1 |
| 23 | 0 | 1 | 1 |
| 24 | 1 | 1 | 1 |
| 25 | 1 | 1 | 1 |
| 26 | 1 | 1 | 1 |
0-Within normal limits;1- Mild to moderate impairment; 2-Severe impairment
Objective 1. To compare the cough pre and post-swallow perceptually: In the present study, it was observed that majority of the participants were rated as having weak cough both pre and post-swallow which is suggestive of overall effectiveness of cough being weak among the stroke individuals [Table/Fig-4,5], except for one of the participants who was observed to have a strong cough both pre and post-swallow. It was also observed that cough sample was mostly rated from very weak to weak post-swallow by both the raters. Although not very evident, there was change of voice observed in the cough sample post-swallow among few of the participants.
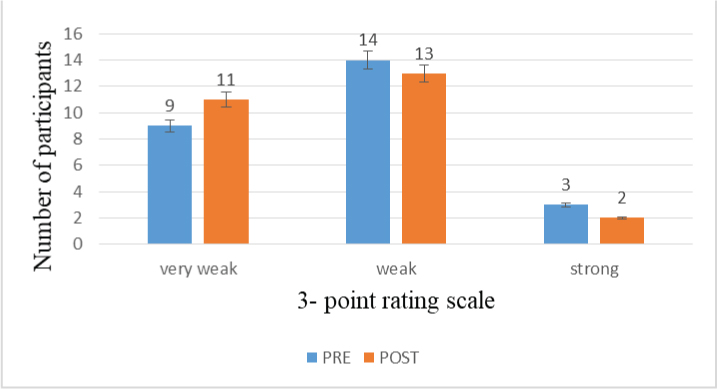
Depiction of perceptual analysis of cough pre and post-swallow by Rater1.
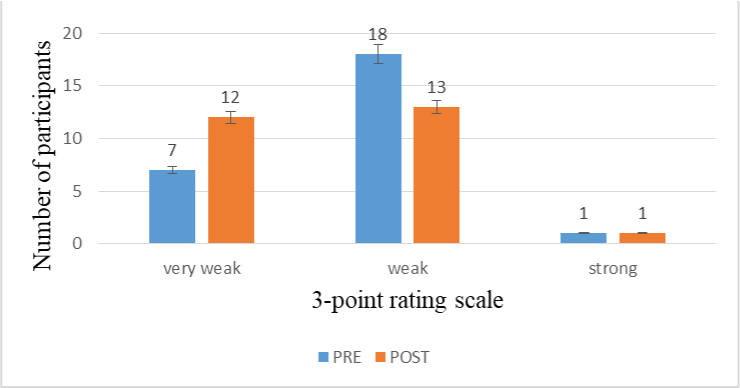
Depiction of perceptual analysis of cough pre and post-swallow by Rater 2.
Inter-rater Reliability
In the present study, Cohen’s Kappa coefficient (κ) was performed in order to determine the agreement between the two raters for perceptual analysis of cough pre and post-swallow. In the [Table/Fig-6] it can be interpreted that there was a moderate agreement between the two raters for perceptual analysis of cough pre-swallow with Cohen’s Kappa (k) = 0.564, and statistically significant p-value (p≤0.001) [Table/Fig-6].
Measure of agreement between both the raters for cough pre-swallow.
| Rater1_pre * Rater2_pre. |
|---|
| Count |
|---|
| Rater2 (pre) | Total |
|---|
| Very weak | Weak | Strong |
|---|
| Rater1(pre) | Very weak | 6 | 3 | 0 | 9 |
| Weak | 1 | 13 | 0 | 14 |
| Strong | 0 | 2 | 1 | 3 |
| Total | 7 | 18 | 1 | 26 |
| Symmetric Measures |
| Value | Asymp. Std.Errora | Approx. Tb | Approx. Sig. |
| Measure of agreement | Kappa | 0.564 | 0.149 | 3.644 | ≤0.001 |
| Number of valid cases | 26 | | | |
There was a moderate agreement between the two raters for perceptual analysis of cough post-swallow with Cohen’s Kappa (k)=0.582, and statistically significant p value (p=0.001) as seen in [Table/Fig-7]. The cough analysis for both pre and post-swallow was observed to be in moderate degree among the raters.
Measure of agreement between both the raters for cough post-swallow.
| Rater1_post * Rater2_post |
|---|
| Count |
|---|
| Rater2 (post) | Total |
|---|
| Very weak | weak | Strong |
|---|
| Rater1(post) | Very weak | 9 | 2 | 0 | 11 |
| weak | 3 | 10 | 0 | 13 |
| strong | 0 | 1 | 1 | 2 |
| Total | 12 | 13 | 1 | 26 |
| Symmetric Measures |
| Value | Asymp. Std.Errora | Approx. Tb | Approx. Sig. |
| Measure ofAgreement | Kappa | 0.582 | 0.150 | 3.443 | ≤0.001 |
| Number of calid cases | 26 | | | |
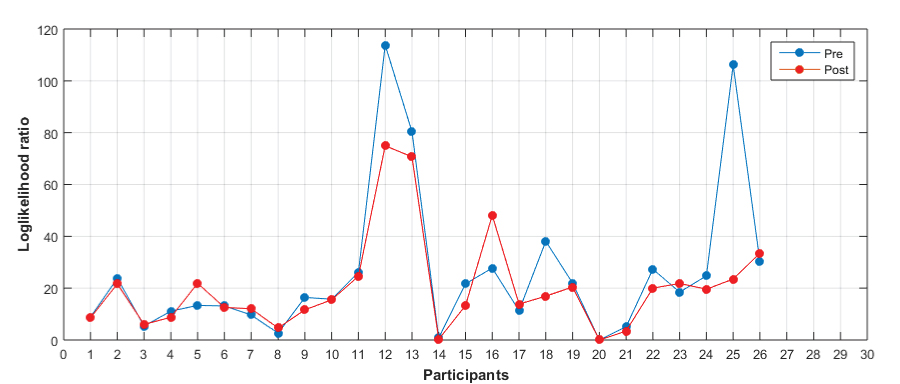
Objective 2- To compare the cough pre and post cough acoustically: The analysis was carried out using the Mel-frequency cepstral coefficients to compare cough pre and post-swallow acoustically. The MFCC plots [Table/Fig-8] indicated that for majority of the participants (n=17), the cough pre-swallow had a higher likelihood value when compared to cough post-swallow. The numerical data derived from MFCC was then compared using the Wilcoxon signed rank test in order to determine the differences statistically. In the present study, the Wilcoxon signed rank test, pre Mdn =17 did not differ significantly with post Mdn=16.5 with Z= -1.352, p=0.177 [Table/Fig-8].
MFCC plots of cough pre and post-swallow.
Discussion
The aim of the current study was to investigate the cough using acoustic analysis in individuals with dysphagia. Earlier studies suggested that lower the scores on FOIS, greater the severity of dysphagia [10]. In the present study, the participants had a FOIS score ranging from Level 2 to Level 5{Level 2(13) to Level 3(9) Level 4 (1) Level 5(2)}, indicating that most of the participants exhibited of having severe dysphagia.
On MMSA, structurally, it was observed that majority of the participants exhibited deviations of lips. Functionally, most of the participants were pooling in the range of mild to moderate impairment, predominantly on the moderate category. On phases of swallowing, (thin liquid consistency; water), it was observed that most of the participants were observed to be in the group of mild to moderate impairment, mostly with moderate impairment. The findings of the present study were in line with the previous study reported by stated that stroke is commonly associated with sensory and motor impairments [11]. Orofacial deficits could also be associated with stroke frequently. It could include asymmetry of face, lips and tongue. They also found laboured mastication, strength of lip seal, poor tongue-palate contact during swallowing.
The other findings observed are absent of gag reflex, acute pain during swallowing, poor oral hygiene, dehydration, loss of weight, delayed oral transit, reduced laryngeal excursion during the feed trial, and gurgly voice post-swallow. Similar findings have been reported by Somasundaram S et al., where authors found individuals with dysphagia had both abnormal gag reflex and volitional cough as well as cough after swallow when compared to non-dysphagic individuals [12]. They also reported that dysphonia, dysarthria and voice change after swallow were less frequently seen in dysphagic individuals which was contrary to the findings of the present study, where in voice change, gurgling was observed post-swallow which is in accordance with Santos KW et al., who reported of significant decrease in grade of voice and asthenia and increase in strain post-swallow of pasty substances [13].
Mann G et al., reported of chronic pain is most often associated with dysphagia [2]. However, in the present study, the participants reported having acute pain during swallowing. Kuhl V et al., reported that laryngeal excursion is reduced in individuals with dysphagia [14]. With respect to the delayed transit, Im Moon H et al., stated that participants with low FOIS scores are more likely to have delayed oral transit which is in consonance with the findings of the present study [15].
In the present study, it indicated that majority of the participants were rated to have weak cough both pre and post-swallow, suggestive of overall effectiveness of cough being weak among the stroke individuals, indicating cough disturbances being more prevalent among Post-stroke individuals. This is in accordance with the incidence of cough disturbances in Post-stroke being about 17.6% to 46.9% [16,17]. Alvares CM et al., attempted to compare the cough pre and post-swallow across varied consistencies among the normal [18]. They observed that the cough was perceived to be stronger and more effective in the normal group.
In few of the participants, it was observed that the cough was perceived weak in the pre cough sample as compared to very weak in the post cough sample. This could be suggestive of residue of food post-swallow and thereby affecting the quality of cough. Although not very evident, there was gurgling observed in the cough sample post-swallow in few of the participants which is again well established in the literature [19]. A perceived change in voice post-swallow has been long considered as an evidence of aspiration of food [20]. It could be postulated that individuals will exhibit changes post-swallow, irrespective of it being speech, voice or cough. Addington WR et al., reported that individuals who exhibited normal cough, Post-stroke were associated with lower risk of aspiration and individuals with weak or an absent cough were associated with significant risk for aspiration [21]. Addington WR et al., emphasised the strength of cough being more crucial in identifying the risk of aspiration pneumonia [6]. They reported that 10% of individuals rated to have weak cough had developed aspiration pneumonia. Further, they also suggested the association between pharyngeal residue and weak cough. They reported that dysphagia in Post-stroke individuals who exhibited weak cough was more likely to have pharyngeal residue. Earlier literature evidence correlated stroke lesions with dysphagia, impaired cough and risk of pneumonia [22]. However, variations in methods, sample size or inaccurate classification of brain regions could have led to inconsistent findings and would have often limited the explanatory power similar to the present study.
The present studies also displayed strong inter-rater reliability between the two raters and it appeared more reliable for rating the cough has weak. It was observed that there was a moderate agreement between the two raters in both cough pre and post-swallow. Similar findings have been reported by Rosenbek JC et al., observed inter-rater reliability to be high between the three experienced raters in all traits of swallowing evaluation clinically, including voluntary cough strength and quality of the cough, wet and dry cough [23]. McCullough GH et al., rated the volitional cough strength by two experienced raters [24]. The volitional cough strength was with 100% agreement as compared to involuntary cough being 85% agreement. The quality of the volitional cough was with 85% agreement and involuntary cough quality with 92% agreement.
However, differing to the previous studies reported, Miles A et al., investigated reliability (intra- and inter) of perceptual assessment of evoked coughs using citric acid as inhalant [16]. The overall perceptual cough assessment demonstrated fair to moderate agreement between the raters. The raters were more consistent in identifying the cough reflex as present or absent as compared to the cough strength. However, it was observed that the agreement was reasonable in identifying the strong coughs as compared to weak cough. Based on the responses observed, the difficulty exhibited was often due to the dearth of knowledge in understanding the description of cough or difficulty in distinguishing between cough and clearing of throat, and or due to lack of expertise in perceptually analysing the cough, similar to the present study.
In the present study, the Wilcoxon signed rank test, pre Mdn=17 did not differ significantly with post Mdn=16.5 with Z=-1.352, p=0.177). Lee KK et al., concluded strong relation between the cough sound and the physiological measures of the cough strength [25]. However, they also reported that microphone placement can have an impact on the measures obtained. Santamato A et al., attempted to measure the swallowing sound post-swallow of varied consistencies [26]. There was significant difference observed for peak intensity, peak frequency and duration of swallow between the healthy and stroke individuals.
Ryu JS et al., reported statistically significant differences in acoustic characteristics post-swallowing among the high risk participants who aspirated as compared to the low risk group [27]. Groves-Wright KJ et al., observed statistically significant difference in acoustic measures that included jitter, shimmer, and noise to harmonic ratio among the individuals who had materials present in the larynx [28]. Therefore, they concluded that any material in the larynx can induce changes in the acoustical characteristics of voice. Murugappan S et al., reported of deviant perturbation measures (high jitter and shimmer measures) in individuals who were observed to have foreign materials (liquid consistency) in the vocal folds [20]. This is in consensus with the findings of the present study which reflected similar findings with changes in the cough post-swallow, although not statistically significant, which could be probably due to small sample size.
Limitation
The recording of cough being restricted to the initial screening; the study could have focused on observing the cough changes over the period of time.
Conclusion
The cough analysis can be incorporated in the bedside evaluation, in identifying the signs of dysphagia. Cough is more easier to elicit as compared to phonation task or speech especially from post-stroke individuals. Since the results of the present study followed a similar trend as that of change of speech or voice post-swallow, cough also can be effectively used in probing the signs of possible aspiration. However, the perceptual analysis may have a bias and therefore confers the need of clincian to be trained in perceptually analysing the sample.
Tube dependent (levels 1-3); 1-No oral intake, 2 -Tube dependent with minimal/inconsistent oral intake, 3 -Tube supplements with consistent oral intake
Total oral intake (levels 4-7); 4 - Total oral intake of a single consistency, 5- Total oral intake of multiple consistencies requiring special preparation, 6- Total oral intake with no special preparation, but must avoid specific foods or liquid items, 7 Total oral intake with no restrictions
0-Within normal limits;1- Mild to moderate impairment; 2-Severe impairment