Primary Localised Amyloidosis of Urethra Presenting as a Penile Stricture
Padmapriya Jaiprakash1, Archana Shivmurthy2, Manna Valiathan3, Arun Chawla4
1 Associate Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2 Associate Professor, Department of Pathology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3 Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
4 Professor and Head of the Department, Department of Urology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Padmapriya Jaiprakash, Associate Professor, Department of Pathology, Basic Sciences, Kasturba Medical College, Madhava Nagar, Manipal Academy of Higher Education, Manipal, Karnataka, India-576104, Karnataka, India.
E-mail: padmapriya.j@gmail.com
Amyloidosis is characterized by the deposition of fibrillar proteins by either plasma cells or derived from circulating proteins, the former especially involved in pathogenesis of localised amyloidosis. We describe a case of primary localised amyloidosis involving the urethra, in a 54-year-old diabetic patient, presenting with symptoms of stricture. Though a rare cause, in a patient with clinical diagnosis of urethral stricture, one of the differential diagnosis to be ruled out is primary urethral amyloidosis.
Fibrillar proteins, Obstruction, Urethroscopy
Case Report
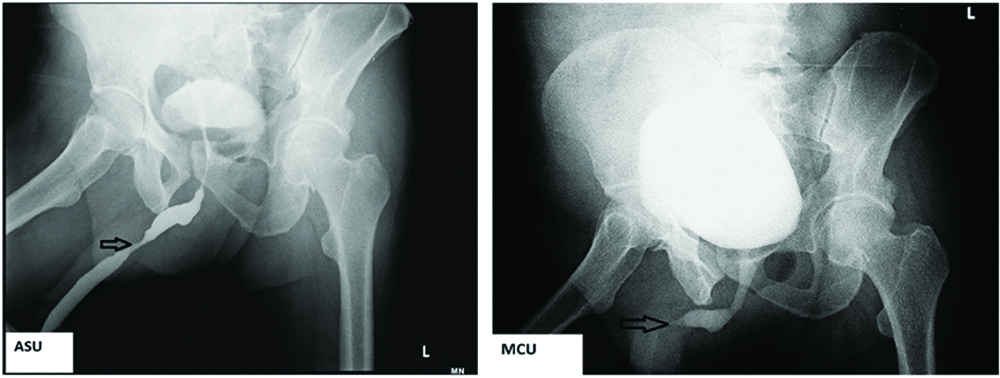
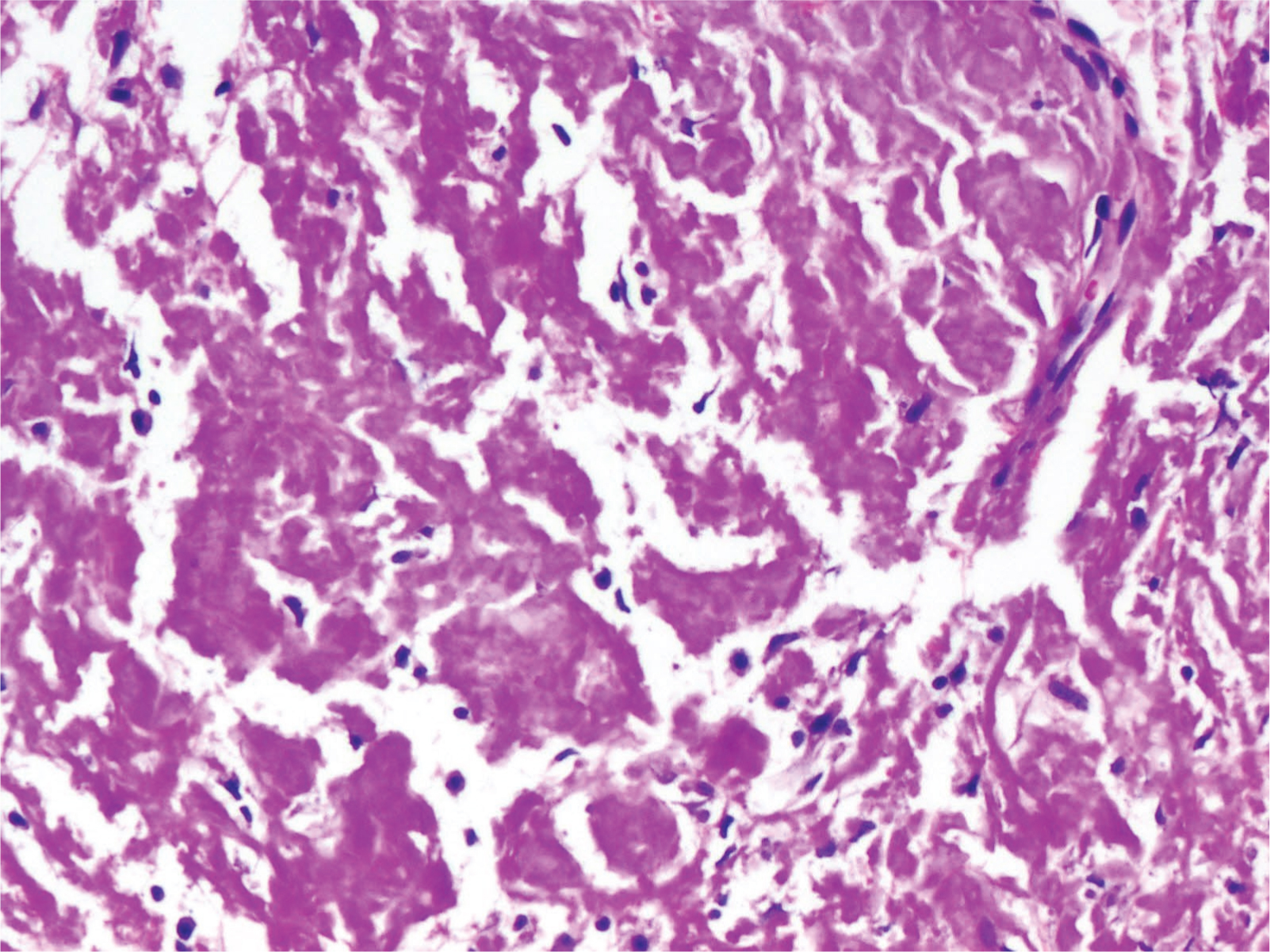
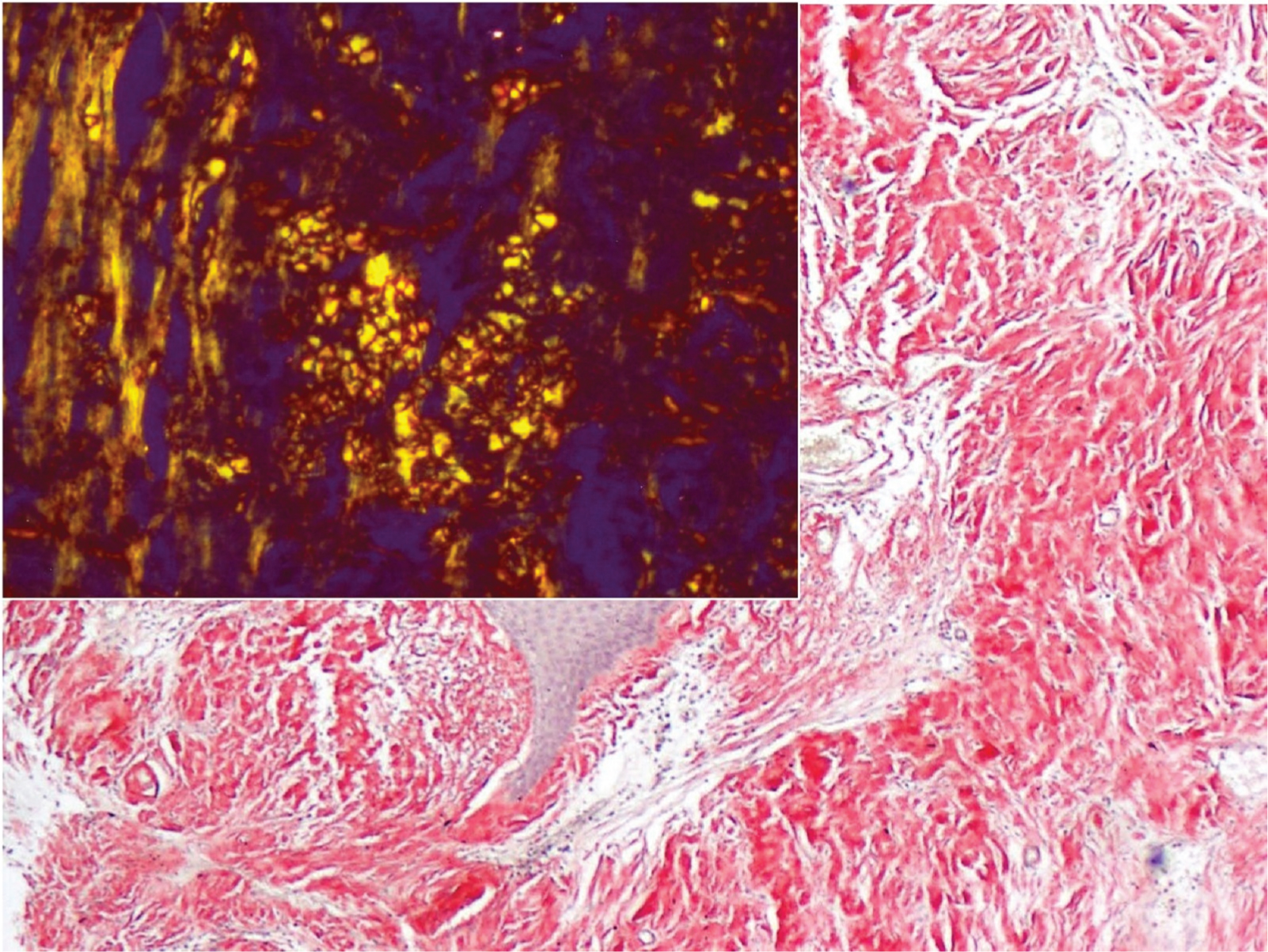
A 54-year-old diabetic male presented to the Urology outpatient with poor stream of urine and dysuria for three months. There was no history of fever or haematuria. He was also a hypertensive. Physical examination revealed no significant findings. Local examination showed grade I prostatomegaly, without any hard or lumpy areas. Urine examination showed 2-4 pus cells and 1-2 red blood cells/high power field (hpf). Urine culture, however, did not yield any organisms. Ultrasound showed normal sized kidneys with distended bladder and normal volume of prostate. An ascending urethrogram [Table/Fig-1a] and micturating cystourethrogram [Table/Fig-1b] showed normal regular outline of urethra with proximal penile urethral narrowing and bladder irregularity, possibly post-inflammatory stricture. The stricture extended almost 2.5 cm from the proximal urethra. A single stage urethroplasty with dorsal and ventral double face graft was done and unhealthy urethral mucosa with stricture, measuring 2x2 cm was removed. Buccal Mucosal Graft (BMG) was harvested from both the cheeks and placed at the excised site. Graft was then fixed to the corpus spongiosum. Excised specimen sent for histopathological examination consisted of flat shaped mucosal bits, which on microscopy showed transitional mucosa with squamous metaplasia, overlying submucosa showing dense deposition of amorphous eosinophilic material surrounded by few foreign body giant cells and lymphoplasmacytic infiltrate [Table/Fig-2,3]. Congo red stain showed pink-red deposits with yellow-green birefringence under polarizing light [Table/Fig-4]. A diagnosis of primary localised amyloidosis of urethra was made, as clinically, no other organ was involved. Investigations done to rule out secondary causes included Erythrocyte Sedimentation Rate (ESR), protein electrophoresis and chest X-ray. Rectal biopsy performed was negative for amyloid deposition. Though the patient was advised bone marrow aspiration and biopsy to rule out myeloma, the patient refused the invasive procedure.
(1a-arrow) Ascending urethrogram showed normal regular outline of urethra with proximal penile urethral narrowing and bladder irregularity; (1b) micturating cystourethrogram.
Transitional mucosa with squamous metaplasia, submucosa showing dense deposition of amorphous eosinophilic material, suggestive of amyloid, surrounded by few histiocytic giant cells (inset-arrow) (H and E, 10x).
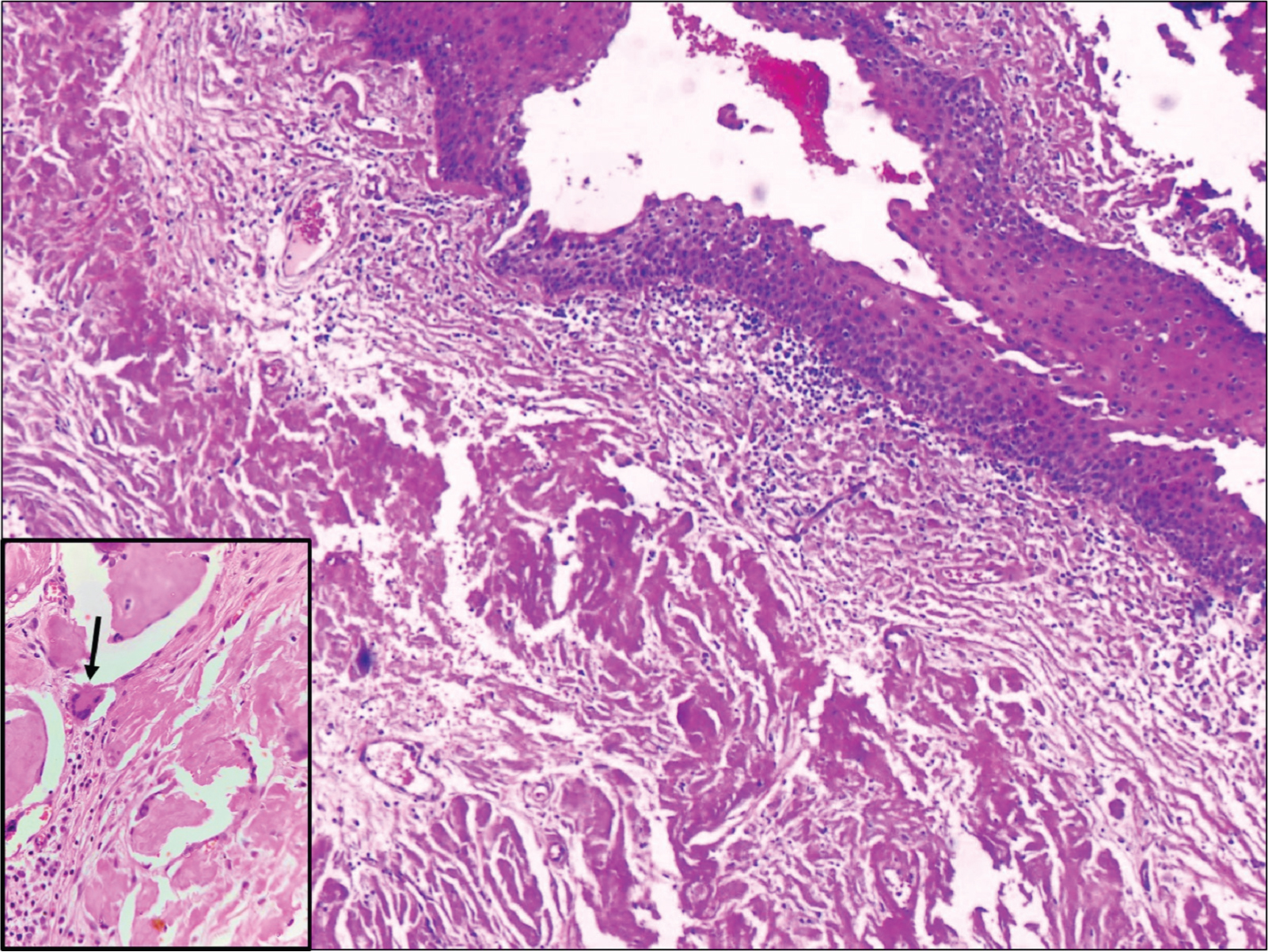
Dense amyloid deposition (H and E, 20x).
Amyloid showing Congo red positivity with apple green birefringence (inset) (Congo red, 20x, 40x).
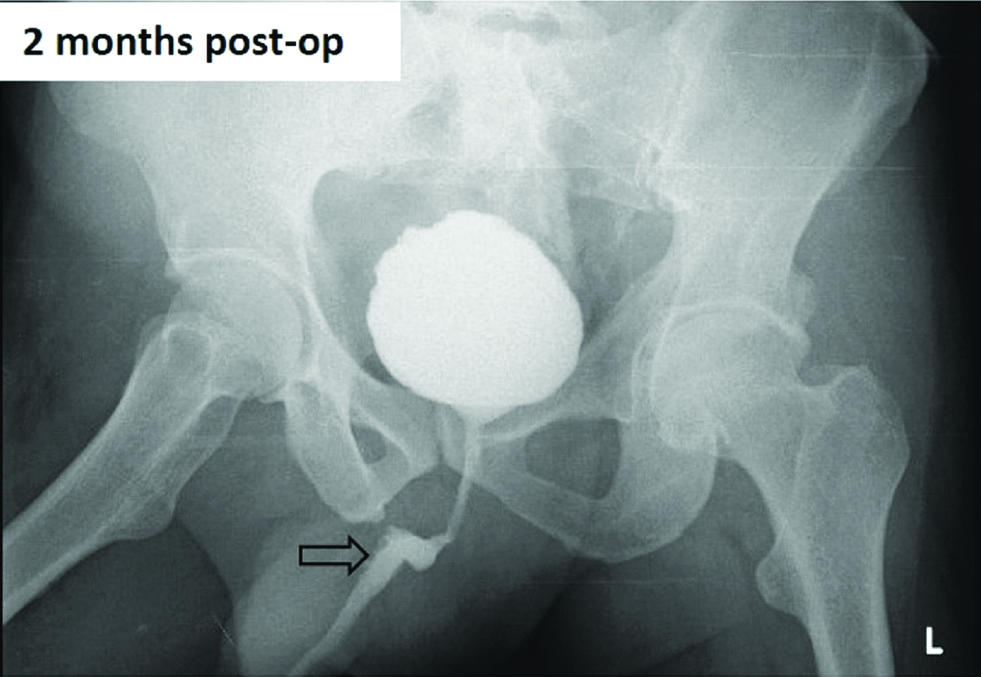
On follow-up, after one, two and six months, the patient was doing well with Micturiting Cysto-Urethrogram (MCU) (at two months) showing good capacity bladder, open bladder neck and good contrast flow in prostatic bulbar and penile urethra, without any extravasation [Table/Fig-5]. He underwent cystoscopy which showed normal penile urethra, with healed anastomotic site having good patency and healthy appearing graft.
Postoperative image showing smooth contour of urethra. (arrow)
Discussion
Amyloidosis is the deposition of fibrillar proteins in the extracellular space which causes tissue damage either mechanically or by tissue dysfunction [1]. Usually seen as a systemic disease, amyloid deposits may be seen in a single organ or tissue, producing gross nodular masses or microscopic lesions, most common sites being lung, larynx, skin, bladder and tongue [1,2].
Tilip A reported the first case of primary localised urethral amyloidosis in the year 1909 [3]. There have been few cases reported in both sexes since then. It has been described in patients ranging from 21 to 82 years of age. Clinically, isolated amyloid deposition in penile urethra and corpus spongiosum may cause painful erection or haemospermia, gross painless haematuria and other non- specific symptoms [3,4]. In a systematic review by Mangera A et al., most of the patients presented with symptoms of stricture [5]. Though cases described in literature are few, the numbers may be deceptive as many of the small fibrotic strictures may not be subjected to biopsy [4,6,7]. Cystoscopic and radiologic findings may be suggestive of a neoplastic process [7-9]. Also, in cases involving the corpus spongiosum alone may not be picked up on urethroscopy. Radiologically, it is associated with irregular narrowing within the bulbous or penile urethra.
Amyloid is associated with plaque-like, punctate or linear submucosal calcifications [5]. In the present case, a clinico-radiologic diagnosis of proximal urethral stricture with a suspicion of malignancy was made. Urethroscopy showed an intact mucosa with subepithelial humps. Deposition of amyloid may be due to chronic or recurrent mucosal and submucosal inflammation, as a part of chronic cystitis. The infiltrating plasma cells secrete an aberrant type of amyloidogenic immunoglobin light chain (kappa, lambda or both) that forms amyloid fibrils and is deposited in the urinary tract [8].
A definite diagnosis is possible only on biopsy or surgical excision with specimens showing apple green birefringence under polarised light after Congo red staining [1,2] with tissue stained by haematoxylin and eosin alone, other eosinophilic deposits like hyaline come into picture. Stains like thioflavin T or S, though nonspecific for amyloid, can highlight small amounts of amyloid which are not stained by Congo Red [4]. Electron microscopy may be used to characterize the deposits especially in renal amyloidosis to differentiate it from various deposits [4]. Immunohistochemistry may be used to find whether deposit is immunoglobulin or not. However, background staining masks the results.
Once a diagnosis of amyloidosis is obtained, a careful search must be made to rule out various causes of secondary amyloidosis, as this condition is associated with poorer outcome [10]. Raja K et al., emphasize this fact and included a battery of tests–urinary and serum protein immunofixation, free light chain assay, electrocardiography, rectal biopsy, to rule out systemic involvement [10]. Investigations to rule out systemic amyloidosis include mucosal biopsies, bone morrow aspiration and biopsy and urine and serum electrophoresis [11]. Thus, the diseases to be excluded include myeloma, chronic infections, familial syndromes and other systemic diseases. The negative history should be supported by negative specific tests and immunostains [11].
Treatment by conservative procedures like urethrotomy and urethral dilatation is associated with high recurrence. Hence, long term solution recommended by Mangera A et al., is complete resection with urethroplasty, which was done in this case [5]. Regarding the type of surgery, Joshi P et al., state that though dorsal onlay can be performed for most strictures, double face urethroplasty is indicated for near-obliterative strictures [12].
Urologists should be aware of this entity and should consider it in the differential diagnosis for patients with haematuria and a mass lesion, especially when no other causes for the mass are identified. Microscopic evaluation of urethral strictures is essential to rule out rare pathologies including amyloidosis. Histological examination of biopsy specimens and staining characteristics including Congo red usually provides the correct diagnosis.
Conclusion
Primary amyloidosis of urethra is an uncommon disease and it should be in the differential whenever clinician is suspecting urethral stricture, as it is the most common presentation or cystoscopic finding of a bulge or mucosal irregularity.
[1]. Diseases of the immune system. In: Kumar V, Abbas AK, Aster JC. (eds.)Robbins and Cotran Pathologic Basis of Disease 2015 9th edPhiladelphiaElsevier Saunders:256-62. [Google Scholar]
[2]. Zhou F, Lee P, Zhou M, Melamed J, Deng F, Primary localized amyloidosis of the urinary tract frequently mimics neoplasia: a clinicopathologic analysis of 11 casesAm J Clin Exp Urol 2014 2(1):71-75. [Google Scholar]
[3]. Tilip A, Ueber lokale tumor termices amyloid der harn rohre centrableE. Allg Path U Path Anat 1909 20:913 [Google Scholar]
[4]. Herrera GA, Turbat-Herrera EA, Differential Diagnosis of Amyloid inSurgical Pathology: Organized Deposits and Other Materials in the Differential Diagnosisof Amyloidosis. In: Picken MM, Herrera GA, Dogan A. (eds.)Amyloid and Related Disorders: Surgical Pathology and Clinical Correlations, Current Clinical Pathology 2015 2nd edChamHumana Press:133-152. [Google Scholar]
[5]. Mangera A, Linton KD, Fernando M, Channer J, Chapple CR, What is the evidence for the management of urethral amyloidosis? A systematic review of the literatureBJU Int 2012 9(12):1858-61.10.1111/j.1464-410X.2011.10635.x22010796 [Google Scholar] [CrossRef] [PubMed]
[6]. Miyamoto SI, Tamiya T, Takatsuka K, Ogawa K, Primary localized amyloidosis of urethraUrology 1919 36:576-78.10.1016/0090-4295(91)80329-6 [Google Scholar] [CrossRef]
[7]. Provet JA, Mennen J, Sabatini M, Rakhman J, Golimbu M, Primary amyloidosis of urethraUrology 1989 34:106-08.10.1016/0090-4295(89)90175-1 [Google Scholar] [CrossRef]
[8]. Noone TC, Clark RL, Primary isolated urethral amyloidosisAbdom Imaging 1997 22:448-49.10.1007/s002619900231 [Google Scholar] [CrossRef]
[9]. Chitale S, Morsey M, Peat D, Webb R, Amyloidosis of lower genitourinary tract: A ReviewEAU-EBU Update Series 2007 5:70-76.10.1016/j.eeus.2006.11.002 [Google Scholar] [CrossRef]
[10]. Raja K, Ahmed E, Mubarak M, Iqbal T, Hassan SM, Primary localized amyloidosis of urinary bladder: a case report and review of literatureNephrourol Mon [Internet] 2013 5(5):994-96.10.5812/numonthly.1087024693509 [Google Scholar] [CrossRef] [PubMed]
[11]. Joo HL, Hoon K, Localized amyloidosis presenting with a penile mass: A case reportCases J 2009 2(10):03-05.10.1186/1757-1626-2-16019946531 [Google Scholar] [CrossRef] [PubMed]
[12]. Joshi P, Kaya C, Kulkarni S, Approach to bulbar urethral strictures: Which technique and when?Turkish Journal of Urology 2016 42:53-59.10.5152/tud.2016.1298927274887 [Google Scholar] [CrossRef] [PubMed]