Older population is increasing in Iran, so that it is predicted that the population above 60 years of age will comprise more than 10% of the total population by 2021, and more than 20% of the population by 2050 [1]. Elderly persons are increasingly at risk for a large number of illnesses. A majority of the elderly (86%) have at least one chronic disease and more than 70% of the elderly above 80 years of age have at least two chronic diseases [2]. About 40% of the elderly in the community experience some restrictions imposed by chronic diseases. The nature of chronic diseases and their long-term course contribute to a large number of symptoms and physical and psychological problems for the elderly [3].
One of the factors playing a prominent role in controlling chronic diseases is patients’ treatment compliance [4]. Medication adherence refers to “the degree to which the person’s behaviour corresponds with the agreed recommendations from a health care provider” [5]. A central principle in medication adherence is the patient’s participation in and acceptance of regular medication utilisation in accordance to prescribed instructions [6]. Since compliance with prescribed medicines is a major challenge for patients with chronic diseases, there are numerous non-adherent patients which may suffer serious complications, including treatment relapse, hospitalisation, and disability [7,8].
The use of a simple, valid, and reliable self-report instrument can lead to a better understanding of medication adherence in the clinical setting. Some instruments have been developed and employed to measure medication adherence. Although these instruments can be beneficial in simple and rapid evaluations of medication adherence, they provide inadequate information due to the limited data obtained (i.e., single dimension or prescription refills) or the scope of the response (i.e., dichotomous response).
The ARMS developed by Kripalani S et al., assesses medication adherence in patients with chronic conditions [9]. The scale assesses both taking medication and refilling medication on schedule. The questionnaire is easy to use, short, and comprehensive and has been used in various studies [10-15]. A major advantage of the ARMS is its suitability for patients with limited literacy skill. Furthermore, few studies have shown that the ARMS may provide a better understanding of adherence to tailor interventions for non-adherent medication-taking behaviours [12,16].
Materials and Methods
Participants and Study Setting
This study used a psychometric design. The research was undertaken in Qom, Iran, from September 2016 to January 2017. In this respect, 100 patients were recruited from the inpatient wards of two university hospitals. The patients were selected as per purposive sampling according to the following inclusion criteria: more than 60-year-old; having a history of chronic diseases for more than six months; using one or more drugs; able to verbally communicate in Persian; have no psychiatric or cognitive disease and willing to participate in this study.
The study procedure was explained to patients who met the eligibility criteria. Ethical approval was granted by the Medical Ethics Committee (MUQ.REC.1396.34), the Qom University of Medical Sciences that corroborated the ethical considerations throughout the study process. Participation in this study was voluntary and participants were informed that they were free to withdraw from the study at any time without having any effect on their treatment process. Those who were willingly agreed to participate in this study signed the informed consent. The data were collected and reviewed by the two researchers. For patients who had difficulty in reading, the researchers read the questionnaire items to the participants. Questionnaire completion took between 10 to 15 minutes.
Measures
For data collection, a three-part questionnaire was used as follows:
Socio-demographic and clinical questionnaire: Patients completed a questionnaire to provide socio-demographic and clinical information such as age, marital status, educational status, employment, economic status, smoking and medical history.
Adherence to refills and medications scale: The original ARMS consisted of 12 items and two subscales: adherence to refilling prescriptions and adherence to taking medication. Kripalani S et al., recommended use of the reduced form of seven items. It is reported that the scale can be used to assess medication adherence of chronically diseased patients with a low level of literacy. Each item was structured for response on a Likert scale with responses of “none,” “some,” “most,” or “all” of the time, which were given values from 1 to 4’. The total score of the shortened seven-item version varies between 7 and 28 [9,19]. Lower scores indicate better adherence. Previous study of the original version of ARMS indicated good reliability and validity [9].
Morisky medication adherence scale: Medication adherence was measured using the MMAS-8 [20]. The scale consists of eight questions, with the first seven items having a yes/no answer (yes=0, no=1) that indicates adherent or non-adherent behaviour. For item eight, a patient can choose an answer on a 5-point Likert scale: 0=Never, 1=Rarely, 2=Sometimes, 3=Often, 4=Always, expressing how often a patient does not take his medications. Possible scores range from 0 to 8, with higher scores indicating better adherence. Validity and reliability of the Persian version of the MMAS-8 has been established [21].
Translation Procedures and Evaluation of Content and Face Validity
The ‘forward-backward’ procedure was applied to translate the scale [22]. For this purpose, at first two independent professional translators were invited to translate ARMS from English into Persian language. The Persian version of the two above translations was obtained with the best translation available. Subsequently in the next step, two English language experts translated this version into English again. After this step, the original English version was compared with the English version derived from the translation of language specialists by the research team, and at last, the final version of the Persian version was approved. After the completion of translation, a multidisciplinary panel of health professionals was assembled to assess the content validity of the questionnaire. They were asked to comment on the reasonability, suitability, attractiveness and logical sequence of items as well as conciseness and comprehensiveness of the questionnaire. Moreover, in order to assess the questionnaire’s face validity, it was given to 10 elders with chronic disease to test its comprehensibility and legibility. This feedback was used to revise the questionnaire with minor wording changes. For example, the phrase of “How often do you run out of medicine” was changed to “How often does your medicine finish.”
Two weeks after the first survey was completed, the second ARMS-SF was re-administered by phone to 10 patients who had agreed to fill in the ARMS-SF again. This procedure was conducted to check the scale reliability using the test-retest method.
Data Analysis
Data analysis was conducted using SPSS version 16 software for Windows. Elder characteristics and the score for each domain of the ARMS-SF were analysed using descriptive statistics. Construct validity of the questionnaire was performed using exploratory factor analysis. The Kaiser-Meyer-Olkin (KMO) and Bartlett’s Test of Sphericity were used to assess the appropriateness of the sample for the factor analysis. Eigen values above 1 and scree plot were used to determine the number of factors. Principal component factor analysis and varimax rotation was used to determine construct validity by excluding items with factor loading of below 0.5 [23]. To assess the criterion-related validity of the ARMS-SF, Pearson’s correlation coefficient between the scores of the ARMS-SF and MMAS-8 were computed. Internal consistency of each subscale of the ARMS-SF was determined using Cronbach’s alpha. A Cronbach’s alpha coefficient of 0.7 or above was considered to be satisfactory. Test-retest reliability was assessed by computing the intraclass correlation coefficient of each domain. The time interval for this assessment was from two weeks in this study with sample of 10. An ICC >0.80 indicated good test-retest reliability [24].
Results
Sample Characteristics
Mean and standard deviation of the patients’ age was 66.46±5.84 years. The sample consisted of 51 percent females and 71 percent of the patients were married. Further information about the personal characteristics of the study participants are presented in [Table/Fig-1].
Socio-demographic and clinical information of the sample (N=100).
| Variables | n |
|---|
| Age (years) | |
| Mean (SD) | 66.46 (5.84) |
| Gender | |
| Males | 49 |
| Females | 51 |
| Educational status | |
| Illiterate | 44 |
| Primary school (<5) | 28 |
| High school (6-12) | 19 |
| Secondary school (>13) | 9 |
| Marital status | |
| Single | 2 |
| Married | 71 |
| Divorced/widowed | 27 |
| Employment status | |
| Employed | 29 |
| Unemployed | 30 |
| Housewife | 41 |
| Income rate | |
| Low | 64 |
| Medium/high | 36 |
| Smoking status | |
| Smoker | 17 |
| Non-smoker | 83 |
| Duration of disease, y | |
| Mean (SD) | 8.58 (8.02) |
| Chronic condition* | |
| Heart disease | 55 |
| Hypertension | 48 |
| Diabetes mellitus | 23 |
| Asthma | 5 |
| Kidney failure Hypertension | 20 |
*Some patients have more than one chronic condition
Validity
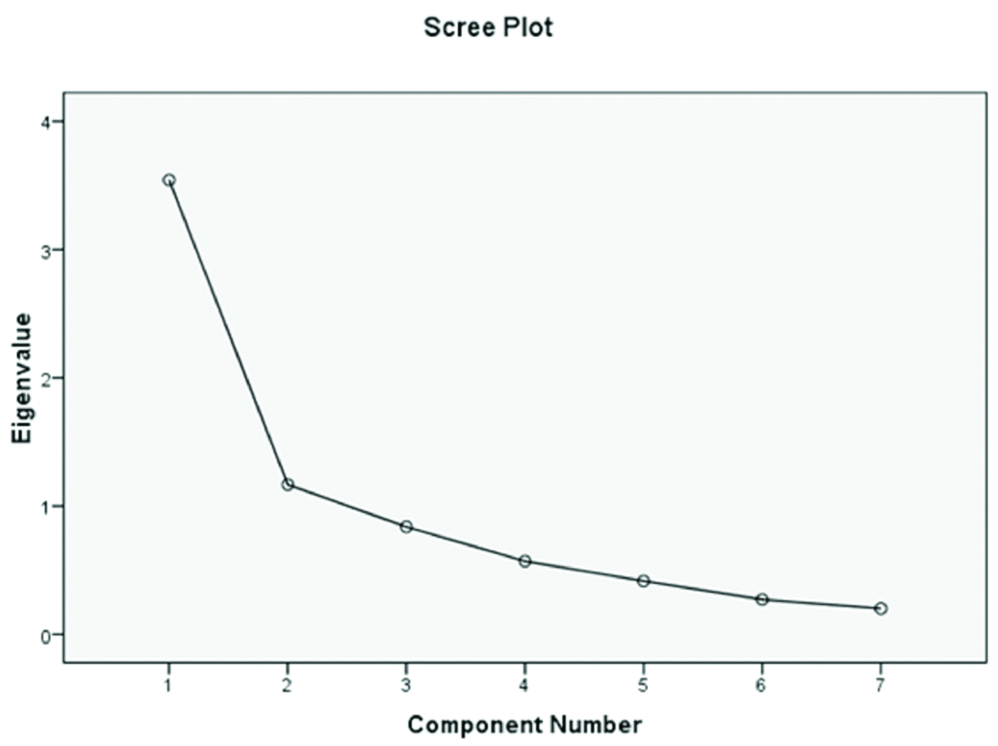
Exploratory factor analysis: The Kaiser-Meyer-Olkin adequacy was 0.76 and Bartlett’s test of sphericity was significant (p<0.0001), showing sampling adequacy. After varimax rotation, the items of scale loaded significantly on two factors. Both factors had an eigen value greater than 1, with an explained variance of 67.26%. A total of items loading ranged from 0.51 to 0.81. The Scree plot suggested generating a two-factor model [Table/Fig-2]. The first factor related to taking medication (five items), and the second factor was refilling medication (two items). Further information about the factor structure is reported in [Table/Fig-3].
Scree plot of the ARMS-SF.
Principal component analysis of the ARMS-P.
| Item | Mean | SD | Factor 1 | Factor 2 |
|---|
| 1-How often do you forget to take your medicines? | 1.65 | 0.83 | 0.810 | |
| 2-How often do you decide not to take your medicines? | 1.38 | 0.69 | 0.818 | |
| 3-How often do you forget to take repeat prescription? | 1.61 | 0.88 | | 0.683 |
| 4-How often does your medicine finish? | 1.66 | 0.68 | 0.728 | 0.528 |
| 5-How often do you not take your medicines because of feeling better? | 1.48 | 0.80 | 0.726 | |
| 6-How often do you not take your medicines because of feeling sick? | 1.29 | 0.64 | 0.628 | 0.513 |
| 7-How often do you plan ahead and refill your medicines before they finish? | 2.54 | 1.07 | | 0.768 |
Factor 1: Taking medication, Factor 2: Refilling medication, Principal component analysis; varimax rotation with Kaiser normalisation; The maximum factor loading of each item is presented with bold value
Criterion-related validity: To test criterion-related validity, correlations among the Persian versions of ARMS-SF and MMAS-8 were measured with the pearson correlation coefficient. ARMS-SF factors and total score demonstrated a significant and moderate levels of correlation with the MMAS-8 score (r=-0.248–-0.506, p<0.05) [Table/Fig-4].
Correlation between ARMS-P and MMAS-8.
| Variable | Taking medication | Refilling medication | Total score | MMAS-8 |
|---|
| Taking medication | 1 | _ | _ | _ |
| Refilling medication | 0.506** | 1 | _ | _ |
| Total score | 0.902** | 0.782** | 1 | |
| MMAS | -0.506** | -0.248* | -0.456** | 1 |
**Correlation is significant at the 0.01 level; *Correlation is significant at the 0.05 level
Reliability
Cronbach’s alpha for the scale was 0.74. For the test-retest reliability, the ICC coefficients ranged between 0.84 and 0.90 for the overall score significance (p<0.001). [Table/Fig-5] shows the internal consistency and the test-retest reliability of the ARMS-SF factors.
Reliability of the ARMS-P.
| Domain score | Item number | Cronbach’s alpha | ICC | p-value |
|---|
| Taking medication | 5 | 0.83 | 0.90 | 0.001 |
| Refilling medication | 2 | 0.51 | 0.84 | 0.001 |
| Total score | 7 | 0.74 | 0.88 | 0.001 |
Discussion
While many studies have been designed and conducted to assess and promote medication adherence in Persian patients [25-27], studies reporting the validity and reliability of Persian medication adherence scales have been lacking. To address this limitation in the literature, the aim of this study was to assess the psychometric properties of the ARMS-SF. The questionnaire was translated based on instrument translation and cultural adaptation guidelines [22]. The face and the content validity of the scale were confirmed after minor revisions.
An exploratory factor analysis method was used to evaluate the construct validity of this questionnaire. The KMO value was relatively high and the Bartlett’s test was significant indicating the appropriateness of the factor analysis model. The scree plot showed that the ARMS-SF consisted of two factors. These factors included ‘Taking medication’, and ‘Refilling medication’. This factor structure is similar to the original structure [9]. The difference between the original and the ARMS-SF was that the Item 4 ‘How often does your medicine finish?’ leads to the ‘Taking medication’ factor instead of the ‘Refilling medication’ Factor. Applying ARMS in different cultures potentially results in discrepancies in its factor structure.
Gökdoğ an F et al., reported a two-factor structure for the Turkish version ARMS [18]. However, Kim CJ et al., reported a structure consisting of three factors in Korean version ARMS-12 including refilling medicine and intentional non-adherence with taking medicine, unintentional non-adherence with taking medicine, and persistence with refilling medicine [17]. These variations in factor structure from language to language highlight the continued need for psychometric study with each ARMS translation.
To establish the criterion-related validity of the ARMS-SF, its correlation to the MMAS-8 was examined. The study findings revealed a significant small-to-moderate level of correlation between the two scales. Previous studies also reported the similar findings [9,17]. There was a good correlation between the ARMS Korean version and 8-item Morisky Medication Adherence Scale-Korean version (r=-0.698) [17].
The study findings also revealed that the ARMS-SF had an acceptable internal consistency. Cronbach alpha coefficient for total, ‘Taking medication’ and ‘Refilling medication’ factors of the ARMS-P was 0.74, 0.83 and, 0.51 respectively. The low Cronbach alpha for the ‘Refilling medication’ factor may be attributed to the small number of items in this sub-scale. Waltz CF et al., noted that the number of items of a measure directly contributes to the magnitude of its Cronbach’s alpha [28]. Additionally, the original study found a Cronbach alpha coefficient of 0.82 for those with a high level of literacy and 0.79 for those with a low level of literacy [9]. Given that the majority of this sample was either illiterate or had less than 5 years of primary school, this low level of literacy may have influenced this study’s slightly slower Cronbach alpha.
Kim CJ et al., assessed the validity and reliability of the Korean version of the ARMS in a sample of diabetic patients and reported Cronbach alpha coefficient of 0.80 [17]. Alpha coefficient in another study conducted by Gökdoğ an F et al., was 0.75 with 100 patients with chronic diseases [18].
In this study, the test-retest reliability of ARMS-SF was satisfactory, when assessing the study participants after a two-week interval. ARMS-SF findings were similar to the original study conducted by Kripalani S et al., and the Gökdoğ an F et al., study [9,18].
Limitation
There are several additional limitations to be noted. Non-random sampling and a small sample size restrict the generalisability of the study findings. Consequently, multi-centre or multi-state studies with larger sample sizes are recommended. Moreover, as we evaluated only the validity and the reliability of the ARMS-SF, conducting studies for assessing the sensitivity to change of the ARMS-P is also recommended.
Conclusion
The results of this study show that the ARMS-SF has good psychometric properties. It is easy for patients to understand and respond and takes less than ten minutes to be completed. This questionnaire can be used to measure adherence taking and refilling medication in elders with chronic diseases, especially those whose literacy level is low.