Amiodarone Induced Fulminant Hepatotoxicity: A Case Report
Saif Alam1, Sumit Singla2, Sanjay Pandit3, Hermanjit Singh Hira4
1 Senior Resident, Department of Medicine, MAMC and Lok Nayak Hospital, New Delhi, India.
2 Associate Proffesor, Department of Medicine, MAMC and Lok Nayak Hospital, New Delhi, India.
3 Associate Proffesor, Department of Medicine, MAMC and Lok Nayak Hospital, New Delhi, India.
4 Director Proffesor, Department of Medicine, MAMC and Lok Nayak Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Saif Alam, Jawaharlal Nehru Marg, New Delhi, India.
E-mail: saifalam83@gmail.com
Amiodarone is an iodine-rich Class III antiarrhythmic drug, which acts via membrane ion channels. It is metabolised in the liver to produce the active metabolite desethylamiodarone. It is highly lipophilic with a large volume of distribution and accumulates in many different tissues, especially the liver. It is widely used in supraventricular and ventricular arrhythmias and given as a loading dose, either via the intravenous or oral route, followed by maintenance doses. The common adverse effects of amiodarone are nausea, vomiting, tremor, thyroid dysfunction, peripheral neuropathy, photosensitivity, bradyarrhythmia, worsening of arrhythmia and the less common ones are pneumonitis, optic neuropathy and hepatotoxicity.
Drug toxicity, Hepatotoxicity, Metabolite
Case Report
A 30-year-old male, recently diagnosed with rheumatic heart disease and severe mitral stenosis. He had severe pulmonary artery hypertension and atrial fibrillation. He was admitted to emergency with complaints of palpitation, shortness of breath, dry cough and easy fatiguability for last 3 to 4 days. He was not on regular treatment. He consume alcohol, amounting to 20-30 grams in a week, for last two years.
On examination, his blood pressure was 96/60 mmHg and pulse was 150 beats per minute, jugular veins were engorged with bilateral pedal oedema. There was no pallor, icterus or cyanosis. His oxygen saturation was 92% on room air. He had bilateral basilar crackles on lung auscultation. His electrocardiogram revealed atrial fibrillation with fast ventricular rate with right bundle branch block pattern [Table/Fig-1]. He was treated with a loading dose of intravenous amiodarone (150 mg over 10 minutes), followed by 300 mg over another six hours. The infusion of amiodarone was continued for the next 24 hours. A total dose of 1350 mg of amiodarone was thus infused. Although, DC cardioversion would have been a better modality for control of his ventricular rate but considering the long duration of atrial fibrillation and the consequent high risk of thrombo-embolism from a dislodged cardiac thrombus, authors opted for intravenous amiodarone. More so, as the facility for emergency transesophageal echocardiography to detect intracardiac thrombus was unavailable in our hospital.
Electrocardiogram of patient showing atrial fibrillation with a rapid ventricular response.
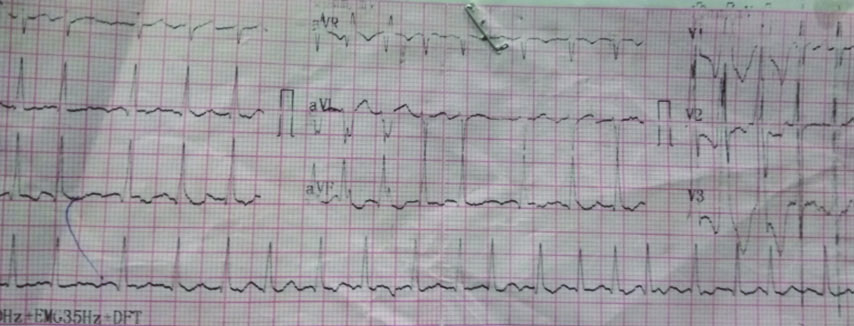
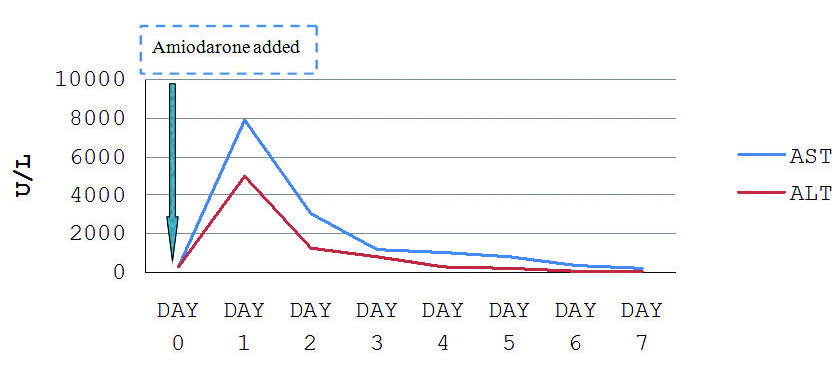
His ventricular rate was subsequently controlled, blood pressure improved over the next 24 hours and congestive heart failure improved with the addition of diuretics. He developed pre-renal Acute Kidney Injury (AKI), which improved with conservative treatment. After 36 hours of admission, on routine evaluation, the patient was found to be icteric. There were no complaints of nausea, vomiting, pain abdomen or fever. There was an abrupt increase in his liver enzymes (AST 7902 U/L, ALT 4973 U/L and ALP 108 U/L), with a conjugated hyperbilirubinemia of 12.6 mg/dL and INR of 2.6 [Table/Fig-2]. On further evaluation, with blood and urine cultures, there was no evidence of sepsis. An urgent ultrasonography of abdomen showed a normal sized liver with normal echotexture, no space-occupying lesion, no intrahepatic bile duct dilatation and there was no evidence of hepatic vein or hepatic artery thrombosis. Serology for hepatotropic viruses and anti-nuclear antibody was negative. He did not have arterial hypoxemia during the course of treatment. Since, other common causes of hepatotoxicity/hepatic injury were ruled out, authors considered it to be case of amiodarone-induced hepatotoxicity. The case scored 6 on the Naranjo Adverse Drug Reaction Probability Scale in the patient [Table/Fig-3].
Laboratory profile of the patient (post-amiodarone infusion).
| Variables | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|
| Total bilirubin (mg/dL) | 1.86 | 12.6 | 9.3 | 8.3 | 8.7 | 6.7 | 5.1 | 3.9 |
| Direct bilirubin | 1.2 | 4.7 | 4.5 | 4.1 | 5 | 3 | 2.3 | 3.3 |
| AST (U/L) | 276 | 7902 | 3061 | 1193 | 1062 | 854 | 376 | 200 |
| ALT (U/L) | 315 | 4973 | 1236 | 843 | 291 | 256 | 99 | 44 |
| ALP (U/L) | 188 | 108 | 191 | 201 | 188 | | | 152 |
| Total protein (g/dL) | 6.21 | 6.8 | | 5.8 | | 5.8 | | 6.4 |
| Albumin (g/dL) | 3.98 | 4.1 | | 2.9 | | 3.2 | | 3.3 |
| PT (second) test | 18 | 34.2 | | 23.7 | | | | 18 |
| INR | 1.6 | 2.6 | 3.9 | 1.852 | | | | 1.3 |
| S. Creatinine (mg/dL) | 0.8 | 1.7 | 1.4 | 1.7 | 1.4 | 1.3 | 1.1 | 1.1 |
| Blood urea (mg/dL) | 33 | 42 | 54 | 54 | 45 | 33 | 34 | 30 |
| Na (mEq/L) | 118 | 121 | 126 | 123 | 135 | 132 | 129 | 132 |
| K (mEq/L) | 5.8 | 4.4 | 5 | 3.9 | 4.9 | 4.5 | 4.6 | 4.5 |
Naranjo adverse drug reaction probability scale.
| S. No. | Question | Yes | No | Do Not Know | Score |
|---|
| 1. | Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | 0 |
| 2. | Did the adverse event appear after the suspected drug was administered? | +2 | -1 | 0 | 2 |
| 3. | Did the adverse reaction improve when the drug was discontinued or a Specific antagonist was administered? | +1 | 0 | 0 | 1 |
| 4. | Did the adverse event reappear when the drug was re-administered? | +2 | -1 | 0 | 0 |
| 5. | Are there alternative causes (other than the drug) that could on their own have caused the reaction? | -1 | +2 | 0 | 2 |
| 6. | Did the reaction reappear when a placebo was given? | -1 | +1 | 0 | 1 |
| 7. | Was the drug detected in blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. | Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. | Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10. | Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | 0 |
| Total Score | 6 |
Total score >9 definite; score 5 to 8 probable; score 1 to 4 possible; score ≤0 doubtful
PT: Prothrombin Time; INR: International Normalized Ratio; AST: Aspartate Transaminase; ALT: Alanine Transaminase; ALP: Alkaline Phosphatase
Amiodarone was immediately withdrawn and the patient improved gradually with progressive normalisation of hepatic function over 7 days [Table/Fig-4]. He was discharged, on day 7, with almost normal liver function tests. On follow up he had normal liver function.
Trend of liver enzymes post amiodarone infusion.
Discussion
Amiodarone-induced hepatotoxicity usually manifests as elevated aminotransferase and alkaline phosphatase levels, documented in less than 3% of patients [1]. These resolve either spontaneously or with dose reduction. The conditions which have a higher risk of amiodarone toxicity are pre-existing liver abnormalities from metabolic syndrome, statin use and heart failure [2].
Acute hepatotoxicity does not occur with therapeutic oral doses of amiodarone [2,3]. A 15-20% of patients may develop raised transaminases (1.5 to 4-fold) following therapeutic doses but only 1% needs drug withdrawal.
The possible mechanisms of chronic amiodarone hepatotoxicity may be inhibition of mitochondrial β-oxidation [4], direct damage to lipid bilayers and disturbance of lysosomes. There are different mechanisms of acute hepatotoxicity following loading intravenous dose, like toxicity due to vehicle polysorbate-80 [5,6], ischaemic liver injury and idiosyncratic toxicity but the exact mechanism is still unknown.
On histopathologic study mallory bodies, steatosis, fibrosis and phospholipidosis are commonly associated with amiodarone-induced hepatitis which is similar to alcohol-induced liver injury [1].
Male gender, underlying cardiomyopathy, congestive hepatomegaly, positive viral markers, increasing baseline total and direct bilirubin, lower serum albumin, increasing random blood sugar, post cardio-pulmonary resuscitation, and DC cardioversion are significant and independent predictors of acute amiodarone toxicity [7]. There is also a linear relationship of acute severe liver injury with baseline aminotransferase level, left ventricular systolic function [7], and advanced age. The underlying compromised cardiac, hepatic and hemodynamic conditions may have predisposed the present patient to amiodarone hepatotoxicity. Present patient had elevated liver enzymes at baseline, congestive heart failure and hypotension, which all of could have predisposed him to acute hepatotoxicity.
Hepatotoxicity may clinically present as cirrhosis and it’s complications with 60% mortality at five months [8]; it may present as cholestasis too [9]. The hepatotoxicity is also frequently associated with renal dysfunction and thrombocytopenia, which usually resolve following withdrawal. The patient in this case also had both hepatotoxicity and acute kidney injury which improved with conservative treatment.
Amiodarone is a life-saving drug; it should be administrated, if indicated, in appropriate dose, although predisposing factors for hepatotoxicity must be sought. Amiodarone is a substrate for CYP3A and CYP2C8 and drugs which that inhibit these isoenzymes, increase serum concentrations of amiodarone to more toxic level. There have been reports of QTc prolongation, with or without Torsades de pointes, in patients taking amiodarone with concomitant fluoroquinolones, macrolide and azole antibiotics. Present patient was not taking any drug which could have had an interaction with amiodarone. In patients with life-threatening arrhythmias, the risk of hepatic injury should be weighed against the potential benefit of amiodarone therapy. Amiodarone should be withdrawn once there is progressive rise of liver enzymes from the baseline but can be restarted safely once liver enzymes normalised. Liver function should be evaluated more frequently when amiodarone is administered intravenously, and at six months after starting oral amiodarone at therapeutic doses for chronic use.
Total score >9 definite; score 5 to 8 probable; score 1 to 4 possible; score ≤0 doubtful
PT: Prothrombin Time; INR: International Normalized Ratio; AST: Aspartate Transaminase; ALT: Alanine Transaminase; ALP: Alkaline Phosphatase
[1]. Pendyala VS, A Case of Amiodarone-induced hepatitis and review of the literatureJ Hepatol Gastroint Dis 2016 2:120 [Google Scholar]
[2]. Pollak PT, How toxic is amiodarone to the liver?J Gastro Liv Dis 2010 19(1):11-13. [Google Scholar]
[3]. Mattar W, Juliar B, Gradus-Pizlo I, Kwo PY, Amiodarone hepatotoxicity in the context of the metabolic syndrome and right-sided heart failureJ Gastrointestin Liver Dis 2009 18(4):419-23. [Google Scholar]
[4]. Fromenty B, Fisch C, Labbe G, Degott C, Deschamps D, Berson A, Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in miceJ Phar-macol Exp Ther 1990 255(3):1371-76. [Google Scholar]
[5]. Ratz Bravo AE, Drewe J, Schlienger RG, Krahenbuhl S, Pargger H, Ummenhofer W, Hepatotoxicity during rapid intravenous loading with amiodarone: description of three cases and review of the literatureCrit Care Med 2005 33(1):128-34.10.1097/01.CCM.0000151048.72393.4415644659 [Google Scholar] [CrossRef] [PubMed]
[6]. Rhodes A, Eastwood JB, Smith SA, Early acute hepatitis with parenteral amiodarone: a toxic effect of the vehicle?Gut 1993[cited 2018 June] 34(4):565-66.10.1136/gut.34.4.5658491409 [Google Scholar] [CrossRef] [PubMed]
[7]. Diab OA, Kamel J, Abd-Elhamid AA, Predictors of intravenous amiodarone induced liver injuryThe Egyptian Heart J [internet .2016[cited 2018 June] 69(1):45-54.Available from: https://doi.org/10.1016/j.ehj.2016.05.00110.1016/j.ehj.2016.05.00129622954 [Google Scholar] [CrossRef] [PubMed]
[8]. Hussain N, Bhattacharyya A, Prueksaritanond S, Amiodarone-induced cirrhosis of liver: what predicts mortality?ISRN Card [internet] 2013 [cited 2018 june] 2013(617943)14 pages. Available from: http://dx.doi.org/10.1155/2013/61794310.1155/2013/61794323577267 [Google Scholar] [CrossRef] [PubMed]
[9]. Macarri G, Feliciangeli G, Berdini V, Jezequel AM, Benedetti A, Canalicular cholestasis due to amiodarone toxicity. A definite diagnosis obtained by electron microscopyItal J Gastroenterol 1995 27(8):436-38. [Google Scholar]