Gliomas are the most common primary brain tumours. They were classified by the WHO in 2007 into; astrocytic tumours, oligodendroglial tumours, ependymal tumours and neuronal andmixed neuronal glial tumours [1].
Despite advances in treatment strategies, gliomas remain one of the most fatal and therapy resistant tumours. Resistance to therapy was attributed to the presence of cancer stem cells (CSCs). These cells are immortal, undifferentiated cells similar to early embryonic cells and they have an exclusive ability to self renew and give rise to tumourigenic cancer cells [2]. They are thought to be the determinants for the occurrence, development, recurrence as well as therapy response of gliomas [3, 4].
Oct4, also known as Oct3 or POU5F1, is a member of the POU (Pit, Oct, Unc) transcription factor family encoded by the POU5F1 gene (6p2113) and was first identified in 1990 [5, 6]. The expression of Oct4 is restricted to pluripotent stem cells and is downregulated when differentiation is initiated during embryonic development. It is undetectable in adult normal tissue [7]. Oct4 is regarded as a gatekeeper at early mammalian development [8] and regulates the self-renewal and pluripotency of human embryonic stem cells (ESCs) [9, 10]. It plays an important role in maintaining cellular plasticity and promoting the self-renewal and proliferation ability of stem cells [8].
Oct4-positive cells identified in cancer represent CSCs and account for the maintenance and propagation of tumours [11, 12]. It was found that inhibition of Oct4 expression in glioma-initiating cells resulted in suppression of tumour formation and also potentiated sensitivity to conventional chemotherapy [13]. Therefore, in the current study, we aimed to examine the immunohistochemical expression of Oct4 in different types of astrocytoma and correlated the expression with the grade of astrocytoma.
Materials and Methods
This retrospective cross sectional study consisted of 66 archived, formalin fixed, paraffin embedded tissue blocks of astrocytoma (12 as grade I, 19 as grade II, 15 as grade III and 20 as grade IV), collected from pathology department, faculty of medicine, Cairo university, through the period from January 2013 to December 2013. The study was approved by the ethical committee of faculty of medicine, Cairo university.
Each paraffin embedded tissue block was recut by rotatory microtome at 5 microns thickness and was mounted on a glass slide and stained with haematoxylin and eosin (H&E) for routine histopathological examination. The astrocytomas were classified according to 2007 WHO classification [1].
Immunohistochemistry
Paraffin embedded tissue sections were cut at 4 microns thickness and mounted on poly-L Lysine coated slides, and were deparaffinized by fresh xylene, followed by rehydration in graded alcohol. Subsequently, blocking of endogenous peroxidase was done by treating the sections with peroxide block for 15 minutes in room temperature, followed by antigen retrieval for 15 minutes in pressure cooker. Then, the sections were incubated with power blocks for 15 minutes, and primary anti-Oct4 antibody (Ventana, 760-4392), ready to use, was incubated for 40 minutes. DAB chromogen was then incubated for 5-10 minutes. Finally, slides were washed and haematoxylin was used as a counterstain.
High power (x400) was specifically used for examining the intensity of staining which was categorized into (weak, moderate and strong). Expression of Oct4 immunostaining was identified by both nuclear and cytoplasmic staining. 5% was used as the scoring point for calculating the percentage of positive cells. Any number of immune-reactive cells with any intensity of staining was considered positive. The percentage of positive cells was calculated as <5% or ≥5% in both low grade (GII and GII) and high grade (GIII and GIV) astrocytomas. No other previous papers mentioned about scoring criteria. Section of testicular seminoma was used as a positive control of Oct4.
Statistical Analysis
Statistical analysis was done using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test or (Fisher’s exact test) was used to examine the relation between qualitative variables. All tests were two-tailed. Only a p-value <0.05 was considered significant.
Results
The 66 biopsies of astrocytoma included in this study were classified according to 2007 WHO classification into; 12 cases of grade I astrocytoma (18.2%), 19 cases of grade II astrocytoma (28.8%), 15 cases of grade III astrocytoma (22.7%) and 20 cases of grade IV astrocytoma (30.3%). The different grades were further subclassified into; 8 cases of pilocytic astrocytoma (12.1%) and 4 cases of subependymal giant cell astrocytoma (SEGA) (6.1%) as grade I-14 cases of diffuse astrocytoma (21.2%) and 4 cases of pleomorphic xanthoastrocytoma (6.1%) as well as one case of pilomyxoid astrocytoma (1.5%) as grade II-15 cases of anaplastic astrocytoma (22.7%) as grade III and finally 16 cases of glioblastoma (24.2%) and 4 cases of gliosarcoma (6.1%) as grade IV. Females represented 52% of the study group.
Out of the 66 biopsies of astrocytoma, 36 (54.5%) were negative for Oct4 and 30 (45.5%) showed positive Oct4 expression [Table/Fig-1]. 10 cases (33.3%) showed nuclear expression and 9 cases (30%) showed cytoplasmic expression, while 11 cases (36.7%) showed both nuclear and cytoplasmic expression. The higher the grade of astrocytoma, the higher was the number of immunoreactive cases (p-value=0.006), as well as the percentage of positive cells in each case (p-value=0.039) [Table/Fig-2]. As for the intensity of immunostaining, it was not much affected by the grade (p-value=0.107) [Table/Fig-3].
Oct4 expression in relation to the grade of astrocytoma (Fisher’s exact test).
| Grade | Oct4 expression | Total | p-value |
|---|
| Positive | Negative |
|---|
| Grade I | 1 (1.5%) | 11 (16.7%) | 12 (18.2%) | 0.006 |
| Grade II | 7 (10.6%) | 12 (18.2%) | 19 (28.8%) |
| Grade III | 8 (12.1%) | 7 (10.6%) | 15 (22.7%) |
| Grade IV | 14 (21.2%) | 6 (9.1%) | 20 (30.3%) |
| Total | 30 (45.5%) | 36 (54.5%) | 66 (100%) |
Percentage of positive cells in relation to the grade (Fisher’s exact test).
| Grades | Percentage | Total | p-value |
|---|
| <5% | ≥5% |
|---|
| Grade I and II | 7 (23.3%) | 1 (3.3%) | 8 (26.7%) | 0.039 |
| Grade III and IV | 9 (30%) | 13 (43.3%) | 22 (73.3%) |
| Total | 16 (53.3%) | 14 (46.7%) | 30 (100%) |
Intensity of immunostaining in relation to the grade of astrocytoma (Fisher’s exact test).
| Grade | Intensity | Total | p-value |
|---|
| Weak | Moderate | Strong |
|---|
| Grade I and II | 5 (16.7%) | 1 (3.3%) | 2 (6.7%) | 8 (26.7%) | 0.107 |
| Grade III and IV | 4 (13.3%) | 7 (23.3%) | 11 (36.7%) | 22 (73.3%) |
| Total | 9 (30%) | 8 (26.7%) | 13 (43.3%) | 30 (100%) |
Discussion
Gliomas account for almost 80% of primary brain tumours and most of them show marked therapy resistance. Therefore, studies are done to detect the pathways involved in their development and progression and the possible implication of these pathways in future improvement of therapy lines.
Despite major improvements in knowledge of the biology of gliomas and the molecular and genetic events implicated in the gliomagenesis, little therapeutic progress has been made in the past years and the survival median for glioblastoma patients remains low [14]. Only a small percentage of cells have the potential to recreate the original tumour to its full heterogeneity, and these cells share phenotypic traits with normal stem cells. These cells have been given the name cancer stem cells (CSCs) [14]. Dysregulation of self-renewal of ESCs plays a key role in generation of CSCs [2,15,16].
Oct4 has been implicated in a variety of cellular functions, including maintaining the pluripotency of ESCs and directing their differentiation to particular cell lineages [16, 17]. Ectopic expression of Oct4 in epithelial tissues causes dysplasia by blocking epithelial stem cell differentiation [16, 18]. In several studies, it was suggested that the adult stem cell, expressing the Oct4 gene, was the target cell to start the carcinogenic process [7,11,19].
In this study, 66 biopsies of astrocytoma were collected and examined histopathologically, and were classified according to 2007 WHO classification [1] to investigate the immunohistochemical expression of Oct4 protein in different grades of astrocytoma.
The mean age for the patients enrolled in this study was 35±19.4 and it ranged between 2 years and 78 years. Females represented the higher group, as we had 34 females (52%), compared to 32 males (48%) of the study group. Subtypes of astrocytoma of higher grades showed a predilection for higher age group (p-value=0.006), but no specific gender preference (p-value=0.671). No statistical relation was detected between Oct4 expression and age or sex. No other comparative previous studies were available in this regard.
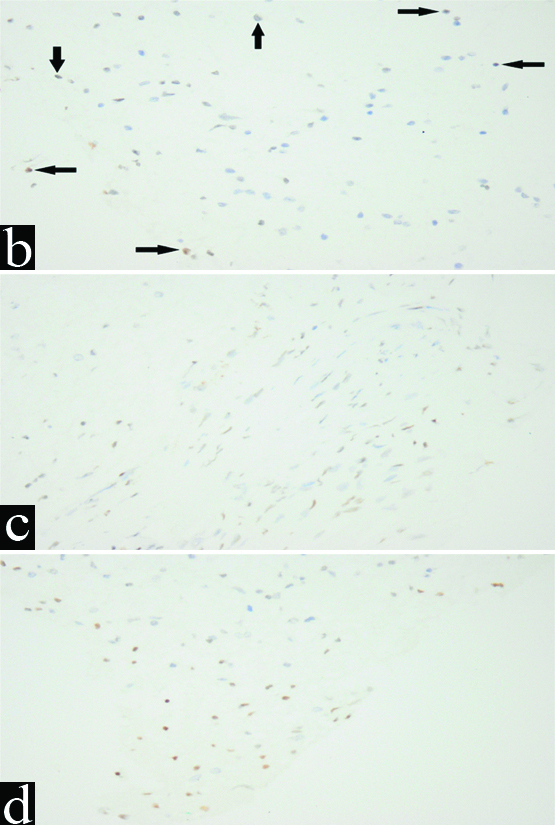
On examination of Oct4 immunohistochemical expression, 36 cases (54.5%) were negative [Table/Fig-4a-d] while 30 cases (45.5%) showed positive expression. The pattern of expression was variable. 10 cases (33.3%) showed nuclear expression [Table/Fig-5a,5b-d], while 9 cases (30%) showed cytoplasmic expression [Table/Fig-6a,b] and 11 cases (36.7%) showed both [Table/Fig-7a-d]. Contrarily, Yuji et al. reported positive expression in 95% of cases, while, coping with the current study, the expression was mainly nuclear, but cytoplasmic expression was also detected [20]. Also, Zhanhui et al. reported expression in all 41 gliomas included in his study and the expression was exclusively nuclear [16]. Oct4 was expressed in all 114 astrocytomas included in the study made by Jeanette et al. They similarly reported that the expression was mainly nuclear, but cytoplasmic expression could be seen too [21]. On the other hand, Oct4 expression was detected only in a few cells in grade IV as reported by Shidou et al. and it was both nuclear and cytoplasmic [22].
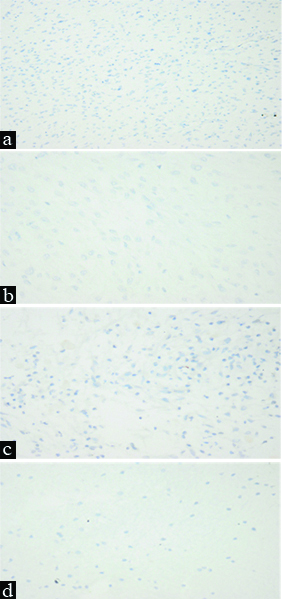
a) Pilocytic astrocytoma, negative staining for Oct4 (IHC, x200); b) Subependymal giant cell astrocytoma, negative staining for Oct4 (IHC, x400); c) Pilomyxoid astrocytoma, negative staining for Oct4 (IHC, x400); d) Diffuse astrocytoma, negative staining for Oct4 (IHC, x400).
Pilocytic asctrocytoma, occasional weakly positive nuclear staining for Oct4 (IHC, x400).

b) Diffuse astrocytoma, weakly positive nuclear staining for Oct4 (IHC, x400); c) Anaplastic astrocytoma, weak nuclear staining for Oct4 (IHC, x400); d) Anaplastic astrocytoma, moderate nuclear staining for Oct4 (IHC, x400).
a) Pleomorphic xanthoastrocytoma, strong cytoplasmic positivity for Oct4 (Arrows) (IHC, x400); b) Anaplastic astrocytoma, occasional moderate cytoplasmic positivity for Oct4 (IHC, x400).

a) Glioblastoma, moderate nuclear (black arrows) and cytoplasmic (white arrows) staining (IHC, X400) b) Glioblastoma, strong nuclear (black arrows) and cytoplasmic (white arrows) staining (IHC, X400); c) Gliosarcoma, moderate nuclear staining (black arrows) and strong cytoplasmic (red arrows) and moderate cytoplasmic (white arrows) staining (IHC, X400); d) Gliosarcoma, strong nuclear staining (black arrows) and strong cytoplasmic (red arrows) and moderate cytoplasmic (white arrows) staining (IHC, X400).

Oct4 intensity of staining was found to be higher in higher grades of astrocytoma. Most cases showed moderate and strong intensity [Table/Fig-5d, 6b, 7a-d]. On the other hand, in low grade astrocytomas, the intensity was predominantly weak [Table/Fig-5a,b], while only 3 cases showed higher intensity [Table/Fig-6a]. Similarly, Zhanhui et al., and Jeanette et al., reported similar results [16,21].
The percentage of positive cells was found to be related to the grade with a significant p-value=0.039, which agreed with the results reported by Zhanhui et al., Yuji et al. and Jeanette et al. [16, 20, 21]. On the other hand, Shidou et al., reported that Oct4 expression was only detected in a few cells in grade IV but not detected in any of the lower grades [22].
As regard the intensity of Oct4 expression, it was widely variable within the different grades in this study, and even within the same case, but it was not much affected by the grade (p-value=0.107). Jeanette et al. reported similar results, while Zhanhui et al. and Yuji et al. reported that the intensity was much higher in higher grades than in low grades [16, 20, 21].
No expression was detected in normal brain tissue in this study, keeping with Zhanhui et al. findings [16].
Limitation
The limitation of this study was, not being able to conduct it according to the new molecular classification of astrocytomas in 2016 WHO classification due to limited resources and lack of references in this regard.
Conclusion
Oct4 is a transcription factor involved in the pluripotency of embryonic stem cells. Its aberrant expression in adult tissues is thought to be involved in the development of CSCs and the development of many tumours including astrocytomas. The expression is higher, the higher the grade of astrocytoma. Further studies should be carried out to detect the effect of suppression of Oct4 on the progress of astrocytomas and the possible benefit of immunotherapy.