Materials and Methods
This is a prospective observational study based on diagnostic accuracy of tests and utilised archived and anonymised serum samples. This work was carried out in the Department of Microbiology, Mahatma Gandhi Medical College and Research Institute (MGMC and RI) Puducherry, during the period January 2017 to May 2018. Patients’ selection was made on the basis of the following inclusion and exclusion criteria:
Inclusion criteria: Presence of typical eschar/fever with or without rash, chills and rigor/hepatosplenomegaly/lymphadenopathy/low platelet count/increased liver enzymes/fever with capillary leak syndrome.
Exclusion criteria: Immunocompromised status/bleeding disorders/Pulmonary tuberculosis etc., [11].
Additionally 70 healthy voluntary blood donors were included for control purpose. Our Institutional Human Ethics Committee (IHEC) approved this research project and granted waiver to retrieve the archived samples and proceed after anonymising the samples by way of excluding patients’ identity (Project No: FACULTY/2015/04 dated 5.06.2015).
ST IgM ELISA
ST IgM ELISA plates were coated with 10 recombinant antigens of Orientia tsutsugamushi, targeting antibodies to the 56-kDa antigen. The kit used was ST Detect IgM ELISA (InBios International, Seattle, USA). Briefly, serum samples were first absorbed with Rheumatoid Factor (RF) sorbent to avoid false positivity and diluted 1:100 with the sample diluent and added to the micro-wells. The plates were incubated, washed, conjugate added and incubated further. Finally, substrate solution was added and the reaction was stopped by adding stop solution and the absorbance was read using an ELISA reader with a wavelength of 450 nm. Test was performed in strict adherence to the procedure outlined in the technical brochure and as described earlier [11,12]. Samples with Optical Density (OD) values of ≥0.5 were taken as positive [13]. OD values of 140 ST IgM ELISA positive samples are listed in [Table/Fig-1].
OD values for ST IgM ELISA Positive samples (n=140).
| Sl. No. | Range of OD Values in ST IgM ELISA | No. of Positive Samples |
|---|
| 1 | ≥0.502-0.700 | 8 |
| 2 | ≥0.701-0.900 | 6 |
| 3 | ≥0.901-1.100 | 34 |
| 4 | ≥1.101-1.300 | 43 |
| 5 | ≥1.301-1.500 | 2 |
| 6 | ≥1.501-1.700 | 5 |
| 7 | ≥1.701-1.900 | 2 |
| 8 | ≥1.901-2.100 | 5 |
| 9 | ≥2.101-2.300 | 2 |
| 10 | ≥2.301-2.500 | 3 |
| 11 | ≥2.501-2.700 | 6 |
| 12 | ≥2.701-2.900 | 2 |
| 13 | ≥2.901-3.000 | 1 |
| 14 | ≥3.001-3.976 | 21 |
| Total | 140 |
ST IgM IFA
ST IFA Kits from Fuller Laboratories (OTM-120 Fuller Laboratories, Fullerton, California, USA) were used. IFA slides were coated with four different prototype antigens of O. tsutsugamushi namely Karp, Kato, Gilliam and Boryong. IFA was performed strictly adhering to the kits’ protocol. Briefly, Patients’ sera were diluted 1:64 in IgM serum diluent, added to the IFA slides and incubated for 30 minutes to allow reaction of serum antibody with four Orientia tsutsugamushi serotypes. Then conjugate was added to label the antigen-antibody complexes, and incubated for 30 minutes. The slides were washed to remove non-reactive serum proteins, washed again to remove non-reactive conjugate, dried and mounted with the mounting medium and read with ×400 magnification, at 390 nm using Primo Star iLED Fluorescent microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany). Positive reactions may then be retested at higher dilutions to determine the highest end point dilution. As per the kit, the cut-off titre of ≥1:64 for IFA IgM were considered positive. Positivity in any one/or more of the four serotypes was taken as IFA positivity.
Statistical Analysis
Based on the 85% sensitivity of the antibody tests, sample size (n) was calculated using the formula:
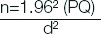
P=0.85 (Sensitivity of the antibody tests)
Q=1-p=0.15
d2=0.06 (Margin of error at 6%).
The sample size was therefore 136 and it was rounded upto 140.
The sample included 140 antibody positive patients in ST IgM ELISA, 70 antibody negative febrile patients with causes other than ST. Additionally 70 healthy voluntary blood donors were included. Thus a total of 280 serum samples were investigated in this study. Sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) between ELISA and IFA were calculated using Graph Pad Quick Calcs (Graph Pad Software Inc, USA) and p-value ≤0.05, was considered as statistically significant. For other parameters, Chi-square and Kappa statistical analysis was performed using IBM SPSS Statistics 17 for Windows (SPSS Inc; Chicago, USA).
Results
Out of 210 febrile patients, 136 were positive for IgM IFA (120 among 140 IgM ELISA positive patients and 16 from 70 ELISA negative patients). Altogether a total of 156 cases were considered as ST patients viz., 120 positive in both ST IgM ELISA and ST IgM IFA, 20 positive only in IgM ELISA and 16 positive only in IgM IFA. All 70 healthy blood donors were negative for IgM antibodies in ELISA as well as IFA. [Table/Fig-2] compares results of ST IgM ELISA vs ST IgM IFA with reference to positivity for Kato/Karp/Boryong/Gilliam serotypes in various combinations. Results of statistical analysis of ELISA vs IFA for IgM is shown in [Table/Fig-3]. IgM ELISA has shown a sensitivity of 88.24% and specificity of 86.11%. Clinical details and laboratory results of 156 patients confirmed as ST by IgM ELSA and/or IgM IFA are presented in [Table/Fig-4], which compares the presentation in children vs adults. Typical ST eschar was observed on 30 patients only (19.23%). There are few parameters such as abdominal pain and malaise that were seen in more number of adults than children (p≤0.05). However, higher number of children had elevated liver enzymes than adults (p≤0.05). Acute Respiratory Distress syndrome (ARDS) and other complications among these 156 cases of scrub typhus are mentioned in [Table/Fig-5].
Comparison of IgM ELISA with various combinations of IFA (n=280).
| Sl. No. | Combination of ST IgM IFA | ST IgM ELISA Positive (n=140) | ST IgM ELISA negative (n=140) | Total (n=280) (%) |
|---|
| 1 | Kato+Karp+Boryong+Gilliam | 60 | 4 | 64 (22.85) |
| 2 | Karp+Kato+Boryong | 2 | 1 | 3 (1.07%) |
| 3 | Karp+Boryong+Gilliam | 4 | - | 4 (1.42%) |
| 4 | Boryong+Gilliam+Kato | 7 | 1 | 8 (2.85%) |
| 5 | Gilliam+Kato+Karp | 5 | - | 5 (1.78%) |
| 6 | Kato+Gilliam | 7 | 1 | 8 (2.85%) |
| 7 | Karp+Gilliam | 3 | 1 | 4 (1.42%) |
| 8 | Boryong+Gilliam | 14 | 2 | 16 (5.71%) |
| 9 | Kato only | 1 | - | 1 (0.5%) |
| 10 | Boryong only | 2 | - | 2 (0.71%) |
| 11 | Gilliam only | 15 | 6 | 21 (7.5%) |
| 12 | Total positive | 120 | 16 | 136 (48.57%) |
| 13 | All Negative | 20 | 124 | 144 (51.42%) |
| Total | 140 | 140 | 280 |
ST IgM IFA Positivity of different serotypes (n=136):
Karp → 80; Kato → 89; Boryong → 97; Gilliam → 130
Sensitivity and Specificity of ST IgM ELISA Vs. ST IgM IFA (n=280).
| Tests | Kappa (95% Confidence Interval) | Sensitivity (95% Confidence Interval) | Specificity (95% Confidence Interval) | Positive Predictive Value (95% Confidence Interval) | Negative Predictive Value (95% Confidence Interval) |
|---|
| ST IgM ELISA Vs ST IgM IFA | 0.743 (0.664 to 0.821) | 88.24% (81.60 to 93.12) | 86.11% (79.37 to 91.31) | 85.71% (79.91 to 90.05) | 88.57% (82.96 to 92.50) |
Clinical findings and laboratory results of acute Scrub Typhus cases: Comparison between children and adults (n=156).
| Clinical/Laboratory findings | Children (0-18 years) (n=52) | Adults (≥19 years) (n=104) | Total (n=156) (%) | p-values* (Chi-square/Fisher’s exact test) |
|---|
| Fever 3 to 7 days | 23 | 48 | 71 (45.51) | 0.8201 |
| Fever 8 to 15 days | 29 | 56 | 85 (54.48) | 0.8201 |
| Chills and Rigor | 22 | 52 | 74 (47.43) | 0.3644 |
| Malaise | 11 | 44 | 55 (35.25) | 0.0091 |
| Headache | 16 | 49 | 65 (41.66) | 0.5092 |
| Myalgia | 19 | 40 | 59 (37.82) | 0.8154 |
| Abdominal Pain | 24 | 72 | 96 (61.53) | 0.0052 |
| Nausea | 12 | 38 | 50 (32.05) | 0.0894 |
| Vomiting | 17 | 41 | 58 (37.17) | 0.4122 |
| Cough and Expectoration | 26 | 48 | 74 (47.43) | 0.6502 |
| Pneumonitis | 5 | 8 | 13 (8.33) | 0.7610 |
| Eschar | 13 | 17 | 30 (19.23) | 0.1961 |
| Lymphadenopathy | 8 | 15 | 23 (14.74) | 0.8731 |
| Hepatomegaly | 9 | 14 | 23 (14.74) | 0.5229 |
| Splenomegaly | 3 | 9 | 12 (7.69) | 0.7519 |
| Rash | 2 | 3 | 5 (3.20) | 0.7479 |
| Pedal oedema | 0 | 5 | 5 (3.20) | 0.1700 |
| Elevated Liver Enzymes (AST/ALT/ALP)† | 9 | 7 | 16 (10.25) | 0.0401 |
| Creatinine (>1.0 mg/dL) | 7 | 18 | 25 (16.02) | 0.4244 |
| Thrombocytopaenia (<1.5 lacs/mm3) | 11 | 19 | 30 (19.23) | 0.6665 |
| Leucocytosis (>11,000 mm3) | 8 | 15 | 23 (14.74) | 0.8731 |
*p-values <0.05 were considered significant; †AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase
Complications of scrub typhus.
| Clinical/Laboratory findings | Children (0-18 years) (n=52) | Adults (≥19 years) (n=104) | Total (n=156) (%) | p-values* (Chi-square/Fisher’s exact test) |
|---|
| ARDS | 2 | 5 | 7 | 0.7845 |
| Acute renal failure | 0 | 1 | 1 | 0.4781 |
| Myocarditis | 0 | 3 | 3 | 0.5363 |
| Hepatic dysfunction | 0 | 1 | 1 | 0.4781 |
| Septic shock | 0 | 2 | 2 | 0.8013 |
Serological response in 156 patients from whom the samples were collected in the first week and second week of febrile illness is presented in [Table/Fig-6]. Higher positivity of 64.10% was observed in samples collected between eight to 15 days, compared to 35.90% positivity in the samples collected during the first week of fever (3 to 7 days). However, the difference is not statistically significant (p=0.1760). Positive reaction was visualised as small sharply defined green fluorescent rods within each antigen spot. Counterstained (red) cells alone seen in negative reaction. Images of IFA positive and negative samples are shown in [Table/Fig-7].
Comparison of results of ST IgM ELISA vs ST IgM IFA (n=210) with reference to duration of fever.
| Category | Fever 3 to 7 days (n=81) | Fever 8 to 16 days (n=129) | Total |
|---|
| Concordance (patients Positive for ELISA and IFA) | 45 | 75 | 120 |
| Concordance (patients Negative for ELISA and IFA) | 25 | 29 | 54 |
| IFA Positive, but ELISA Negative | 5 | 11 | 16 |
| ELISA Positive, but IFA Negative | 6 | 14 | 20 |
| Total | 81 | 129 | 210 |
ST IgM positive and negative cases.

Discussion
ST has become an endemic disease in Puducherry and surrounding Tamil Nadu, necessitating routine testing for ST in all cases of acute febrile illnesses. ST IgM IFA kits of Fuller Laboratories, USA has been validated and found to be satisfactory by overseas and Indian researchers [14-17]. This is also the experience with this kit, on the basis of this research. Unlike ELISA, IFA is a quantitative test, subjective in nature, demand good technical experience and expensive. ELISA is a qualitative test, but it is objective, does not require high technical expertise and is cost effective. The major drawback with the gold standard IFA is the prevalence of diverse O.tsutsugamushi serotypes in different parts of the world. The complete profile of O.tsutsugamushi genotypes circulating in India is yet to be compiled. This might lead to a poor sensitivity of IFA inspite of its remarkable specificity.
Sensitivity of IFA varies from 42% to 100% according to different authors. Thus, Kim DM et al., observed a sensitivity of 42% in blood samples collected before seven days of fever, and steadily increasing to 76%, 84%, 96% in second, third and fourth weeks of illness [18]. Blacksell SD et al., reported 69% and 86.20% in admission and paired samples respectively [19]. La Scola B et al., demonstrated a sensitivity of 84%, 70%, 48% and 54% with IgM IFA cut-off titres of ≥1:100, ≥1:200, ≥1:400 and ≥1:1200 respectively [1]. Lim C et al., Coleman RE et al., and Stephen S et al., reported sensitivity of 81.5%, 85% and 75% respectively [10,20,21]. Recently Pote K et al., observed that at a cut-off titre of ≥1:64, Fuller labs ST IgM IFA kit has a sensitivity of 96.8% [16]. Phetsouvanh R et al., noted a sensitivity of 95% [22]. Gupta N et al., and Wongchotigul V et al., reported a sensitivity of 100% at a cut-off titre of 1:64 and ≥1:400 respectively [15,23].
Performance of ST IgM InBios ELISA kit was found to be equal to IFA IgM Kit [24] or some times better than the gold standard IFA [16]. In the present study, against the gold standard IFA, ELISA has a Kappa factor of 0.743, which is considered to be ‘good’. Cut-off titres for ELISA and IFA must be considered for different geographical regions. ST IgM ELISA InBios kit’s cut-off OD values for India has been set at 0.5 [13], while some authors consider 0.8 [25] and 1.0 [17]. Regarding IgM IFA test, the cut-off titres of ≥1:64 for IFA IgM was considered as per the recommendation of the kit. Positivity in any one/or more of the four serotypes Kato, Karp, Boryong and Gilliam was taken. The seropositivity of ST patients to more than one serotype indicates antigenic cross reactivity among them. For serological diagnosis of acute ST, presence of IgM antibody is considered, since IgG positivity might point to past infection/secondary infection. Significant ST IgM IFA titre of ≥1:64 were considered by Pote K et al., recently from Central India [16]. As per STIC, serological confirmation of ST can be made by any one of the following two conditions [9]:
IgM IFA titre in acute samples should be ≥1:10,240
or
Seroconversion/four-fold increase in IgM IFA titres in paired sera (≥1:10,240).
To demonstrate seroconversion/four-fold increase in titre of IFA, convalescent samples from patients is required. However, patients coming from rural and remote areas may find it difficult to come again for the second visit. IgM ELISA is not included in STIC, for being a qualitative test. This is inspite of the fact that the ELISA is still considered as a viable alternative to the highly subjective, technically demanding and expensive IFA, because of its simplicity, affordability and availability in India [11-13,15-17,19]. STIC is yet to get universal approval. Modified STIC by Lim C et al., has substantially lowered the IgM IFA titre from ≥1:10,240 to ≥1:3,200 [10]. Even this titre is not reached by Indian patients as reported by many Indian rickettsiologists and their cut-off titres ranged from 1:64 [15,26], ≥1:128 [14,17] to ≥1:256 [27]. Kim DM et al., from Korea and Tantibhedhyangkul W et al., from Thailand considered IgM/IgG IFA cut-off titres of ≥1:400 or a fourfold increase in convalescent samples as significant [18,28]. According to Sonthayanon P et al., a single IFA IgM titer of ≥1:400 or a 4-fold or greater rise in IFA IgM titre points to ST [29]. Therefore, there is an urgent need to address this issue and define the optimal cut-off titre in countries like India so as to understand the true prevalence of ST in different geographical regions. Incorporation of local strains of O. tsutsugamushi in the serological kits would help in identifying more cases of ST.
There are instances of false positivity with ST IgM ELISA due to other febrile diseases like Typhoid, Malaria, Leptospirosis, Dengue and Pulmonary tuberculosis [30,31]. The earlier understanding was that in ST patients IgM antibody appears early, stays at significant level only for about a month whereas IgG appears late during second or third week of febrile illness and persists for several months [31]. However, recent report by Varghese GM et al., changed this concept as they have demonstrated the persistence of IgM and IgG antibodies in ST patients’ upto 13 and 36 months respectively [25]. Hence in endemic areas, ST IgM positivity has to be taken with caution and requires clinical correlation. Molecular tests aimed at detecting O.tsutsugamushi DNA would be helpful in confirming present infection.
Limitation and Future Recommendation
A total of 280 participants were tested. Larger number of cases and controls might perhaps give a clear picture of ST prevalence in this region. ST IgM IFA kits need to be made available at affordable cost and efforts should be made to manufacture them in India, incorporating local isolates of O. tsutsugamushi.
Conclusion
ST has to be included in the list of acute febrile illnesses in India. ST IgM IFA kit needs further evaluation throughout India so as to decide the optimal cut-off titres in different geographical locations. Until this is achieved, perhaps the best alternative and only viable option available for India is to continue with ST IgM ELISA using appropriate cut-off OD values adjusted to the respective geographical regions.
ST IgM IFA Positivity of different serotypes (n=136):
Karp → 80; Kato → 89; Boryong → 97; Gilliam → 130
*p-values <0.05 were considered significant; †AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase