A Comparative Study on the Efficacy of i.v. Cyclophosphamide Pulse Versus Oral Cyclophosphamide in Bullous Pemphigoid
Rajkumar Kannan1, Jayakalyani Vijayananth2, Muthusubramanian Chandrasekar3
1 Associate Professor, Department of Dermatology, Chengalpattu Medical College, Chengalpattu, Tamil Nadu, India.
2 Senior Resident, Department of Dermatology, Chengalpattu Medical College, Chengalpattu, Tamil Nadu, India.
3 Senior Resident, Department of Dermatology, Chengalpattu Medical College, Chengalpattu, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rajkumar Kannan, Flat-C, New No.3, Athipoo Flats, Thiruvalluvar Street, Methanagar, Chennai-600029, India.
E-mail: rajderm0002@gmail.com
Introduction
Bullous pemphigoid is an acquired autoimmune blistering disorder characterised by subepidermal bullae and deposition of complements and antibodies along the basement membrane zone. It most commonly affects the elderly and hence is frequently associated with co-morbidities. Corticosteroids have been the mainstay of treatment.
Aim
To compare the efficacy and safety of i.v. Cyclophosphamide pulse versus oral cyclophosphamide in patients of bullous pemphigoid.
Materials and Methods
The study was conducted in the Department of Dermatology at a tertiary care centre from May 2017 to April 2018. A diagnosis of bullous pemphigoid was made based on the clinical and histopathological findings. A thorough history and detailed physical examination was done. Patient’s demographics, co-morbidities and treatment history were recorded. A total of 15 patients were chosen for the study. They were randomly divided into two groups. Both groups were started on tab. Prednisolone 1 mg/kg/day. In group A eight patients were started on i.v. Cyclophosphamide pulse 500 mg intravenous, at 28 days interval. In group B seven patients were started on tab. Cyclophosphamide 50 mg/day. Patients were followed with periodical complete haemogram, liver function tests and urine analysis monthly during treatment and for a period of 3 months after achieving remission.
Results
I.v. Cyclophosphamide pulse was found to have shorter duration of treatment and lesser cumulative dose when compared to oral cyclophosphamide.
Conclusion
I.v. Cyclophosphamide pulse proves to be an effective drug for bullous pemphigoid than daily oral cyclophosphamide, but warrants close monitoring.
Pulse therapy, Recalcitrant bullous pemphigoid, Steroid sparing
Introduction
Bullous pemphigoid is an acquired autoimmune blistering disorder characterized by subepidermal bullae and deposition of complements and antibodies along the basement membrane zone. It most commonly affects the elderly and hence frequently associated with co-morbidities [1]. The pathognomonic sign of bullous pemphigoid includes sub-epidermal blisters, lesional and peri lesional polymorphonuclear cell infiltrates in the upper dermis and Immunoglobulin (Ig) G and C3 bound to dermo-epidermal junction [2]. The treatment of bullous pemphigoid is aimed at decreasing blister formation and improving the quality of life. Corticosteroids have been the mainstay of treatment [3]. In recalcitrant lesions, in patients with severe co-morbidities where steroids are contra-indicated and to reduce the high dose and duration of steroid exposure, other immunosuppressives like azathioprine, mycophenolate mofetil, methotrexate and cyclophosphamide can be used [3]. But these drugs have adverse effects of their own. Cyclophosphamide has been known to cause haemorrhagic cystitis and malignancies [4,5]. These adverse effects can be reduced with pulse dosing. Apart from the presence of circulating and tissue bound autoantibodies against BP180 and BP230 of IgG subclass, serum levels of IgE type of BP180-NC16A antibodies, were associated with more severe forms of human bullous pemphigoid and it has been found out from research that the serum titre of the later co-related well with the disease activity of corticosteroid-resistant bullous pemphigoid, where it had a direct implication on the extent of cutaneous lesions [6-8]. Probably patients with this particular IgE type of BP180 antibodies experienced the so called recalcitrant variety of bullous pemphigoid and this particular study focuses on the cost-effective cum minimal adverse effect prone treatment strategies. Cyclophosphamide is one of the most effective steroid sparing alkylating agent, that can be given both orally and parenterally, as pulse dosing as well as on a daily basis and hence the present study focuses on the comparison of parenteral versus oral dosing of this alkyating agent, in the treatment of recalcitrant bullous pemphigoid.
Materials and Methods
This is a prospective open-labelled interventional study conducted in the Department of Dermatology at Chengalpattu Medical College, Chengalpattu from May 2017 to April 2018. After obtaining clearance from the Institutional Ethical Committee (31/2017/IEC-CMCH), patients of more than 18 years of age were chosen for the study. A written and informed consent was obtained from the patients before including them in the study. A diagnosis of bullous pemphigoid was made based on the clinical and histopathological findings. A thorough history and detailed physical examination was done. Patients demographics, co-morbidities and treatment history were recorded. All the baseline investigations including complete haemogram, renal and liver function tests, blood glucose levels were recorded. Patients on other immunosuppressive drugs, with renal failure or with active tuberclosis and pregnant and lactating women were excluded from the study. A total of 15 patients with confirmed diagnosis of bullous pemphigoid were chosen for the study. They were divided into two groups by simple random sampling. Both groups were started on tab. Prednisolone 1 mg/kg/day. In group A eight patients were started on i.v. Cyclophosphamide pulse 500 mg at 28 days interval. In group B seven patients were started on tab. Cyclophosphamide 50 mg/day. Patients were followed with periodical complete haemogram, liver function tests and urine analysis monthly during treatment and for a period of 3 months after achieving remission. The efficacy and safety of the cyclophosphamide in both groups was evaluated.
Statistical Analysis
Appropriate descriptive and inferential statistical analysis was done.
Results
A total of 15 patients from 45-74 years of age were included in the study. The pre-treatment and post-treatment response to i.v. cyclophosphamide pulse and oral cyclophosphamide is given in [Table/Fig-1,2] respectively. The demographic details are given in [Table/Fig-3]. The mean cumulative dose of cyclophosphamide and treatment duration and adverse effects of the drugs in group A and group B is given [Table/Fig-4]. I.v. Cyclophosphamide pulse was found to have shorter duration of treatment and lesser cumulative dose when compared to oral cyclophosphamide.
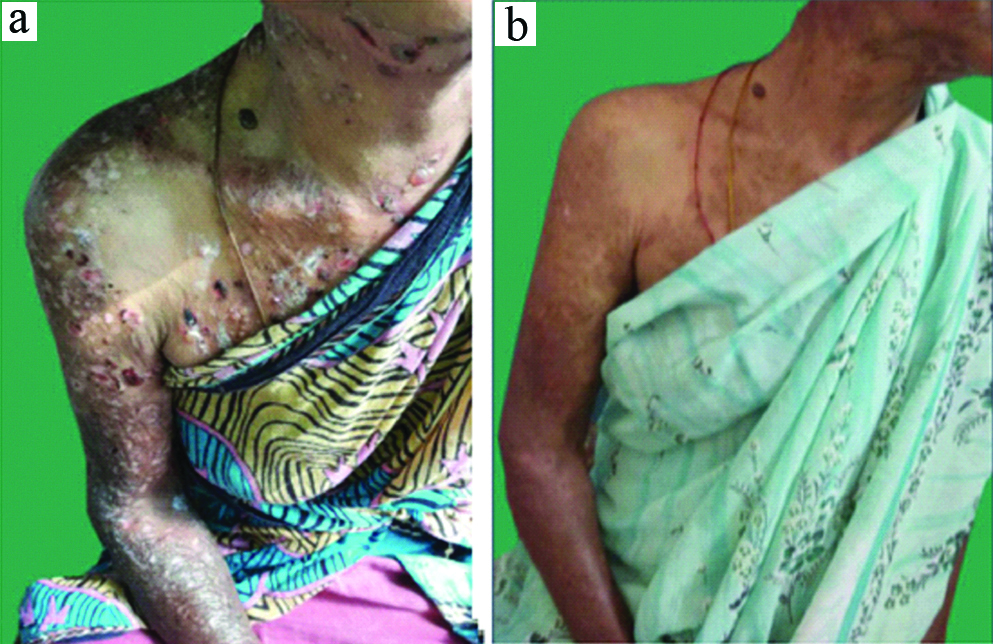
a) Pre treatment-extensive erosions; b) Post 2 cycles i.v. cyclophosphamide pulse.
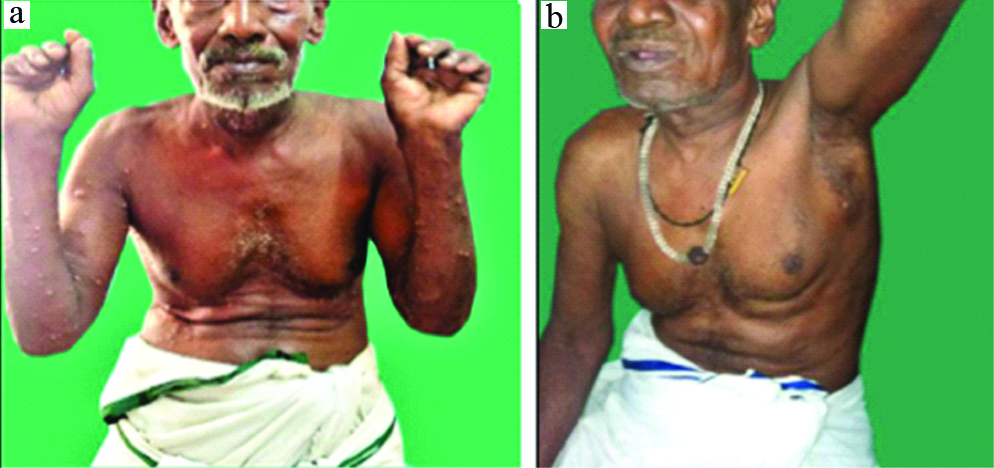
a) Bullae over both forearms and abdomen; b) Post oral cyclophosphamide (14 weeks).
| Group A | Group B |
|---|
| Age (mean) | 64.75 | 61 |
| Male | 5 | 5 |
| Female | 3 | 2 |
| Diabetes mellitus | 4 | 3 |
| Hypertension | 2 | 1 |
| Hypothyroidism | 1 | 0 |
| COPD | 0 | 1 |
| Group A | Group B |
|---|
| Cumulative dose (mean) | 1500 mg (1000-2500) | 5043 mg (4500-6000) |
| Treatment duration (mean) | 12 weeks | 13.7 weeks |
| Adverse effects | No. of patients | No. of patients |
| Nausea | 1 | 2 |
| Vomiting | 0 | 1 |
| Infection (cellulitis, impetigo) | 2 | 0 |
| Dysuria | 1 | 1 |
Discussion
Bullous pemphigoid is an auto-immune subepidermal blistering disorder. It more commonly affects elderly. It is the most common blistering disorder in most countries. Studies regarding the epidemiological data are lacking in India. But in India, pemphigus vulgaris is found to be more common than bullous pemphigoid [9].
The clinical features include pruritic tense vesicles and bullae over an erythematous base or normal skin. Immunologically, autoantibodies against BP230 and BP180 antigen are found [10]. Bullous pemphigoid is diagnosed based on the clinical, histopathological and immunological findings [11,12].
The treatment of bullous pemphigoid depends on the age of the patient, severity of the lesions and associated co-morbidities. Cyclophosphamide is an alkylating agent that acts by cross linking DNA resulting in apoptosis [13]. In dermatology, cyclophosphamide is FDA approved only for mycosis fungoides [14]. In the liver, cyclophosphamide is converted to 4-hydroxyl-cyclophosphamide and aldophosphamide. Aldophosphamide is converted to phosphoramide mustard (active metabolite) and acrolein which causes cell damage [15]. The adverse effects of cyclophosphamide include bone marrow suppression, haemorrhagic cystitis, nausea, vomiting, diarrhoea, carcinoma bladder, amenorrhoea and azoospermia [4]. Various studies have shown that daily dosing of cyclophosphamide kills both normal cells and lymphoma cells whereas in pulse doses only lymphoma cells are affected [4]. With pulse dosing, the incidence of bone marrow suppression could be reduced.
The risk of malignancy in patients of dermatological indications is far lower than the risk in post-transplant and oncology patients. Even though the only FDA approved dermatological indication for cyclophosphamide is advanced mycosis fungoides, cyclophosphamide plays a crucial role in the off-label dermatological indication of bullous disorders both in suprabasal and subepidermal disorders. This is exemplified by the fact that Dexamethasone Cyclophosphamide therapy (DC pulse) has shown to be very effective at limiting the disease activity while minimising corticosteroid adverse effect, in cases of pemphigus vulgaris [16,17]. The fact that it suppresess B-cells more than T-cells, affects T-suppressor cells more than T-helper cells, and last but not least it doesn’t induce myelosuppression, to produce an immunosuppressive effect, makes it a wonderful drug in the treatment of bullous pemphigoid with an added advantage of steroid sparing effect [18].
It was observed in the present study that the cumulative dose of i.v. cyclophosphamide with monthly pulse doses was low, when compared with continuous oral therapy and this reduces the incidence of secondary malignancies [19]. Intermittent high dosage of cyclophosphamide impairs immune surveillance for relatively short periods compared with daily therapy and malignant potential of the same would be lower, particularly urinary bladder malignancies and gonadal toxicity [19]. Also, the previous studies have shown greater improvement of bullous pemphigoid with i.v. Cyclophosphamide pulse than with daily steroids [20]. To our knowledge, there are no studies comparing oral cyclophosphamide and cyclophosphamide pulse in bullous pemphigoid.
Limitation
The Present study is limited by small sample size and a shorter duration of follow up. Controlled trials with larger cohort and longer duration of follow up to establish the efficacy and safety of the pulse dosing should be done in future.
Conclusion
The Present study has shown that i.v cyclophosphamide pulse has lesser cumulative dose and shorter duration of treatment when compared to daily oral cyclophosphamide. This proves to be an effective adjuvant drug for bullous pemphigoid, but warrants close monitoring.
[1]. Gudi VS, Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east ScotlandBr J Dermatol 2005 153(2):424 [Google Scholar]
[2]. Jordon RE, Basement zone antibodies in bullous pemphigoidJAMA 1967 200(9):751 [Google Scholar]
[3]. Mutasim D, Therapy of autoimmune bullous diseasesTher Clin Risk Manag 2007 3(1):29-40. [Google Scholar]
[4]. de Jonge ME, Huitena ADR, Rodenhuis S, Beijnen JH, Clinical pharmacokinetics of cyclophosphamideClin Pharmacokinet 2005 44:1135-64. [Google Scholar]
[5]. Medved A, Maxwell I, Intermittent cyclophosphamide in pemphigus vulgaris and bullous pemphigoidCMA Journal 1974 111:245-5.0. [Google Scholar]
[6]. Haase C, Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2J Invest Dermatol 1998 110(3):282 [Google Scholar]
[7]. Zillikens D, A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoidJ Invest Dermatol 1997 109(5):679 [Google Scholar]
[8]. Hofmann S, Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomainJ Invest Dermatol 2002 119(5):1065 [Google Scholar]
[9]. Khandpur S, Verma P, Vesiculobullous disordersIndian J Dermatol 2011 77(4):450-55. [Google Scholar]
[10]. Labib RS, Anhalt GJ, Patel HP, Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblottingJ immunol 1986 136(4):1231-35. [Google Scholar]
[11]. Zhao C, Murrella D, Advances in understanding and managing bullous pemphigoidF1000 Res 2015 4:1313 [Google Scholar]
[12]. Di Zenzo G, Della Torre R, Zambruno G, Bullous pemphigoid: from the clinic to the benchClin Dermatol 2012 30:03-16. [Google Scholar]
[13]. Fleischli M, Valek R, Pandya A, Pulse intravenous cyclophosphamide therapy in pemphigusArch Dermatol 1999 135(1):57-61. [Google Scholar]
[14]. Thappa DM, Rakhesh SV, Newer concepts in intravenous glucocorticoid and cyclophosphamide pulse therapy in dermatologyIndian J Dermatol 2003 48:125-32. [Google Scholar]
[15]. Blomgren H, Hallstrom M, Possible role of acrolein in 4-hydroxyperoxy cyclophosphamide-induced cell damage in vitroExper Clin Pharmacol 1991 13:11-14. [Google Scholar]
[16]. Mahajan VK, Sharma NL, Sharma RC, Twelve-year clinic-therapeutic experience in pemphigus: a retrospective study of 54 casesInt J Dermatol 2005 44:821-27. [Google Scholar]
[17]. Sacchidanand S, Hiremath NC, Nataraj HV, Revathi TN, Rani S, Pradeep G, Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, BangloreDermatol online J 2003 9:02 [Google Scholar]
[18]. Hall AG, Tilby MJ, Mechanisms of action of and modes of resistance to alkylating agents used in the treatment of haematological malignanciesBlood Rev 1992 6:163-73. [Google Scholar]
[19]. Pretorius E, Davids MR, Toit du R, Oral v. Pulse intravenous cyclophosphamide: A retrospective analysis of adverse events in a setting with a high burden of infectious diseaseSAMJ 2015 105(3):207-13. [Google Scholar]
[20]. Fox LP, Pandya AG, Pulse intravenous cyclophosphamide therapy for dermatologic disordersDermatol Clin 2000 18:459-73. [Google Scholar]