A Study on Microbial Flora on Skin of Health Care Providers in a Tertiary Care Hospital in Southern India
Mathavi Sureshkumar1, R Suriyapraba2, R Indra Priyadharsini3
1 Associate Professor, Department of Microbiology, VMKVMC, VMRF (DU), Salem, Tamil Nadu, India.
2 Student, VMKVMC, VMRF (DU), Salem, Tamil Nadu, India.
3 Professor and Head, Department of Microbiology, VMKVMC, VMRF (DU), Salem, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mathavi Sureshkumar, Associate Professor, Department of Microbiology, Vinayaka Mission’s Kirupananda Variyar Medical College, Vinayaka Missions Research Foundation (Deemed to be University), Salem-636308, Tamil Nadu, India.
E-mail: drmathavimicro@gmail.com
Introduction
Human skin is constantly covered with microorganisms both commensals and pathogens depending on topography, environmental factors and host factors. Though these organisms are regarded as commensals in immunocompetent individuals, they can become pathogenic in immunocompromised persons especially in hospitalised individuals. In recent years, Staphylococcus epidermidis is regarded as an agent of hospital and community acquired infections. When the health care workers do not wash their hands between patients or do not practice standard infection control measures, they are responsible for transmission of nosocomial infections.
Aim
This study was done to know the microbial flora on skin of Health Care Workers (HCWs) and to create awareness on the effective infection control measures.
Materials and Methods
This prospective study was done in Vinayaka Mission’s Kirupananda Variyar Medical College, Salem for a period of four months. Two swabs were collected from 130 Health Care Workers (HCWs) (Doctors, Staff nurse, Medical students, Lab technicians and Housekeeping staff) and subjected to bacterial and fungal culture. Blood agar and MacConkey agar was used for bacterial culture and Sabouraud’s dextrose agar for fungal culture. The bacterial isolates were then subjected to antibiotic sensitivity testing.
Results
Bacterial growth was observed in all the HCWs (100%) and fungus was isolated from eight HCWs (6.2%). Among the bacterial isolates, Diphtheroids (47) were the predominant isolate accounting for 29% followed by coagulase negative Staphylococcus (39) which was 24% of isolates. The predominant pathogen isolated was Staphylococcus aureus (15%, 25 isolates). 17% (11 isolates) of Staphylococci were resistant to Cefoxitin which indicates both MRSA and MR-CONS. Among gram-negative isolates, most of them were resistant to ampicillin, cotrimoxazole and cephalosporins. Candida (4 out of 8, 50%) was the predominant fungal isolate.
Conclusion
Physical contact with HCWs is the most frequent mode of transmission of nosocomial infections. Hence, awareness should be created among HCWs about the significance of handwashing and use of personal protective equipment to prevent cross-infection.
Bacterial flora, Contact transmission, Fungal flora, Handwashing, Nosocomial infection
Introduction
Human skin is constantly covered with microorganisms, both commensals and pathogens depending on topography, environmental factors and host factors [1]. Cultures from the skin have frequently demonstrated bacteria such as Diphtheroids, Staphylococcus,Streptococcus viridans, Streptococcus faecalis, Micrococcus, Corynebacteria, Brevibacteria, Propionibacteria, gram positive aerobic spore-bearing bacilli, gram negative bacilli such as Escherichia coli, Proteus and other intestinal organisms, non-pathogenic Mycobacteria and fungi like Candida albicans and Pityrosporum ovale [1,2]. Hair frequently harbours Staphylococcus aureus and forms a reservoir for cross-infection [3,4].
Most of these organisms act as commensals but they become pathogenic in persons with compromised immune response as in hospitalised patients. In recent years, Staphylococcus epidermidis is regarded as an agent of hospital and community acquired infections. They act as causative agent of bloodstream infections, urinary tract infections and surgical site infections. Another concern is increasing incidence of drug resistance in these organisms. Penicillin resistant Staphylococcus are seen in individuals working in hospitals. The skin of health care providers harbors commensals as well as pathogens including drug resistant organisms due to exposure to hospital environment [5,6]. When these organisms are present on the skin of HCWs they act as source of nosocomial infection [7,8]. When these HCWs do not wash their hands between patients or do not practice standard infection control measures, they are responsible for transmission of nosocomial infections [9]. Once the microbial flora of these HCWs are known, awareness can be created about the hygienic measures and infection control measures to prevent the spread of nosocomial infections.
Effective hand-washing to decrease the transient contaminant flora in the skin is recognised as a critical factor in infection control policies [10]. There are three different techniques of hand washing described in literature: (i) the social hand wash, which is removal of dirt, soil, and various organic substances by use of plain, non-medicated bar or liquid soap and water; (ii) the hygienic (Europe) or antiseptic (United States) hand wash, which is done with antimicrobial or medicated soap (usually containing a single active agent) and water (“scrub”); and (iii) the hygienic hand disinfection (Europe), with the use of an alcohol-based hand rub into dry hands without water [11].
Use of personal protective equipment (gloves, mask, apron, cap and goggles) also plays an important role in protecting the HCWs as well as in reducing the transmission of microorganisms by the HCWs. Adherence to hand hygiene practices is considered as an integral part of quality health care.
This study was done with the following objectives:
To know the bacterial and fungal flora on the skin of HCWs.
To know the antibiotic susceptibility pattern of the bacterial flora.
To create awareness among the health care workers regarding hand washing and other infection control measures to prevent nosocomial and cross-infections.
Materials and Methods
This prospective study was done in Department of Microbiology in Vinayaka Mission’s Kirupananda Variyar Medical College, Salem for a period of four months from September-December 2017. Ethical clearance was obtained prior to conduct of the study (VMKVMC/IEC/17/28). A total of 130 HCWs were subjected to study after getting informed consent. Two swabs were collected from each health care provider for bacterial and fungal culture. Samples were collected from various sites especially from the web space of hand and foot. Blood agar and MacConkey agar was used for bacterial culture and Sabouraud’s dextrose agar for fungal culture. The bacterial colonies were identified by gram staining and biochemical reactions and antibiotic susceptibility testing done according to standard protocol [12,13]. The fungal colonies were identified by gram staining and Lacto Phenol Cotton Blue mount [14].
Results
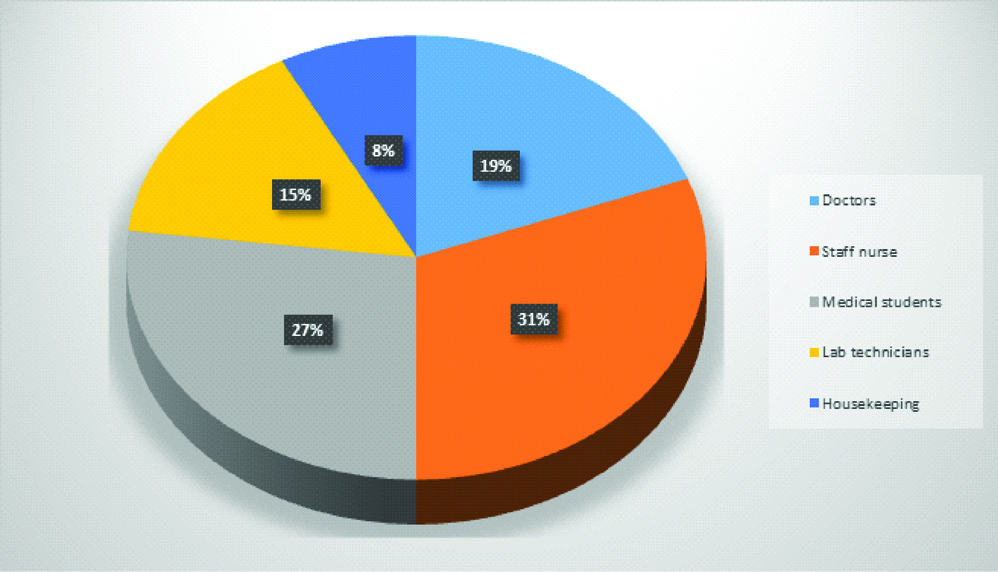
Samples were collected from all groups of HCWs like doctors, medical students, Staff nurse, Lab technicians and housekeeping staff who have contact with patients [Table/Fig-1]. Two swabs were collected from each HCW and subjected for bacterial and fungal culture.
Distribution of health care workers (n=130).
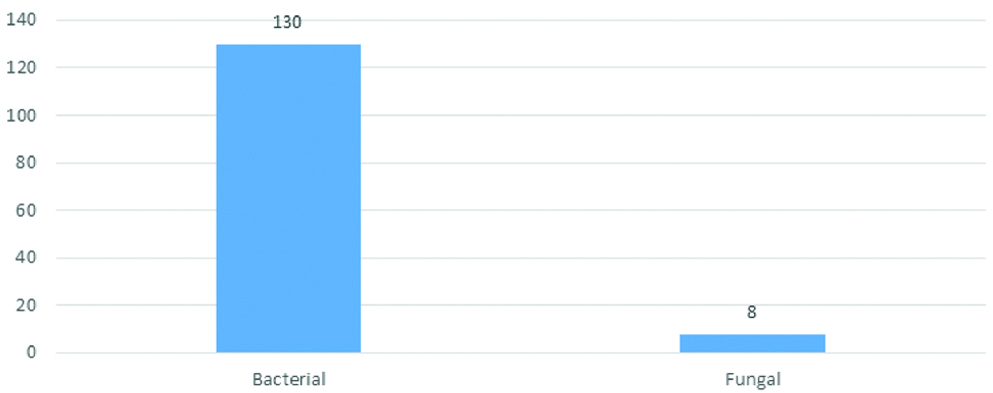
Among the 130 health care workers subjected for study, bacterial growth was observed in all of them (100%) and fungus was isolated from eight HCWs (6.2%) [Table/Fig-2].
Distribution of microbial growth among health care workers (n=130).
A total of 164 organisms were isolated from 130 HCWs. Non-pathogenic bacteria like diphtheroids, CONS and Micrococcus were predominantly isolated. Some also showed the presence of pathogenic bacteria like S.aureus, P.aeruginosa and other Gram negative bacilli [Table/Fig-3]. Candida was the predominant fungus [Table/Fig-4].
Distribution of bacterial isolates (n=164).
| S. No | Organisms | Total isolates |
|---|
| 1 | Staphylococcus aureus | 25 (15%) |
| 2 | Coagulase negative Staphylococcus | 39 (24%) |
| 3 | Diphtheroids | 47 (29%) |
| 4 | Micrococcus | 12 (7%) |
| 5 | Escherichia coli | 9 (6%) |
| 6 | Klebsiella species | 6 (4%) |
| 7 | Proteus species | 4 (2%) |
| 8 | Pseudomonas species | 22 (13%) |
Distribution of fungal growth (n=8).
| S. No | Organisms | Total isolates |
|---|
| 1 | Candida species | 4 (50%) |
| 2 | Mucor | 1 (12.5%) |
| 3 | Aspergillus species | 2 (25%) |
| 4 | Microsporum species | 1 (12.5%) |
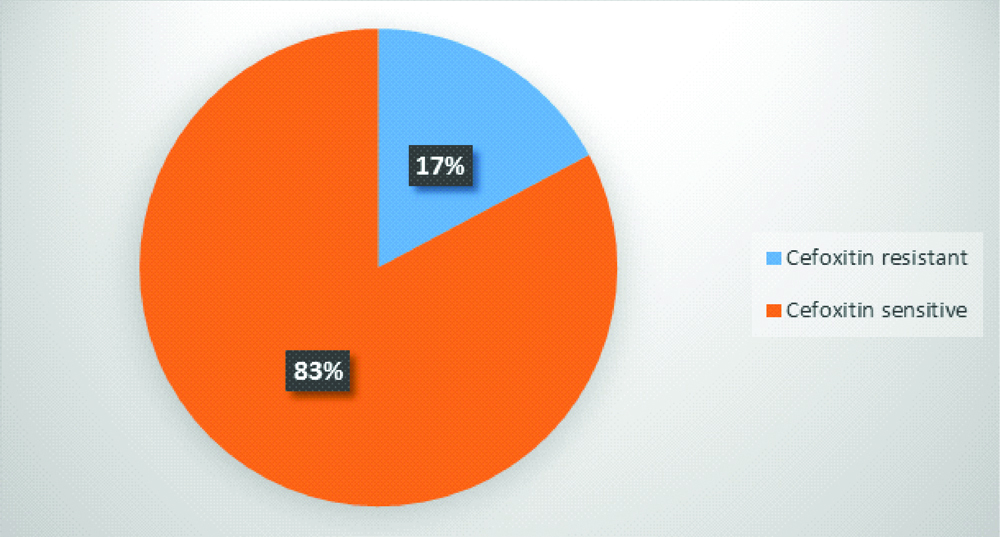
Most of gram-negative bacilli were resistant to ampicillin and cotrimoxazole. All the isolates were sensitive to fourth generation cephalosporins and carbapenems [Table/Fig-5]. 17% (11 isolates) of Staphylococcus were found to be methicillin resistant [Table/Fig-6].
Antibiotic resistance pattern of Gram Negative Bacilli (n=41).
| S. No | Antibiotics | E. coli (9) | Klebsiella (6) | Proteus (4) | Pseudomonas (22) | Resistance (%) |
|---|
| 1 | Ampicillin | 3 | 6 | 3 | 18 | 73% |
| 2 | Cotrimoxazole | 1 | 3 | 2 | 20 | 63% |
| 3 | Cefatoxime | 0 | 2 | 2 | 7 | 27% |
| 4 | Cefepime | 0 | 0 | 0 | 0 | 0% |
| 5 | Cefaperazone/Sulbactum | 0 | 0 | 0 | 0 | 0% |
| 6 | Ciprofloxacin | 0 | 0 | 2 | 6 | 20% |
| 7 | Amikacin | 0 | 1 | 1 | 3 | 12% |
| 8 | Meropenem | 0 | 0 | 0 | 0 | 0% |
Prevalence of Methicillin resistant Staphylococcus among HCWs (n=64 isolates).
Discussion
The increased incidence of nosocomial infections is due to poor infection control practices among HCWs. Adherence to standard precautions by health care providers may reduce the risk of obtaining occupational infection from both known and unexpected source in health care settings [9].
In the present study among HCWs, bacterial growth was observed in all the HCWs (100%) and fungal growth was observed in eight HCWs (6%). The skin of HCWs was found to be colonised with one or more bacterial isolate. This correlates with the study conducted by Sarfraz A et al., which also showed bacterial colonisation in majority of HCWs [15].
Among the bacterial isolates, Diphtheroids (47) were the predominant isolate accounting for 29% followed by CONS (39 isolates, 24%). Apart from the normal commensals, pathogenic bacteria were also isolated from few HCWs. Among the pathogens, predominant isolate was Staphylococcus aureus (25) constituting 15% of the isolates followed by Pseudomonas species (22 isolates, 13%), Escherichia coli (9 isolates, 6%), Klebsiella species (6 isolates, 4%) and Proteus species (4 isolates, 2%). This is very similar to the study conducted by Khodavaisy S et al., among health care workers in ICU which reported that 73% of HCWs hands were contaminated with at least one pathogen [16].
Among the fungal isolates, Candida (4 out of 8, 50%) was the predominant isolate. Other fungi contaminating the skin of HCWs include Mucor (1 isolate, 12.5%) and Aspergillus (2 isolates, 25%). Microsporum (1 isolate, 12.5%) was also found in one HCW. This correlates with study conducted by Khodavaisy S et al., and Brunetti L et al., which also showed contamination with Candida and Aspergillus [16,17].
The present study also shows that 17% of Staphylococcus isolates were resistant to Cefoxitin which indicates both MRSA and MR-CONS are colonising the skin of HCWs, thereby increasing the chance of cross-infection with highly resistant organisms. This is similar to the study done by Sarfraz A et al., [15]. But another study conducted by Kokate S et al., on HCWs showed all the Staphylococcus isolates were sensitive to Cefoxitin [18].
Among gram-negative isolates, most of them were resistant to ampicillin and cotrimoxazole. Resistance was also observed to third generation cephalosporins and fluoroquinolones though at a lower rates. All the isolates were sensitive to Carbapenems, fourth generation cephalosporins as well as to β-lactamase inhibitor combination.
The human skin harbors about 1012 microbes which include both commensal as well as pathogenic organisms. Pathogenic organisms from colonised and infected patients can be carried from one patient to another by HCWs. This could be due to lack of proper infection control measures like hand washing and lack of awareness among the HCWs [15]. Hand washing reduces the level of contaminating flora by 2 to 3 log10 [19]. Al-Busaidi S has proved that proper hand washing by the HCWs reduces the risk of nosocomial infection [20].
HCWs are more prone to acquire drug resistant microbial infections and serve as transmitting vectors of such fatal infections which may be difficult to treat. There is need for training on hand washing and hand disinfection practices among various categories of HCWs for prevention of nosocomial infections.
Limitation
The change is the microbial flora after handwashing was not documented in this study.
Genetic relatedness of the isolates with those isolated from patients cannot be established as genotyping was not done.
Antifungal susceptibility testing was not done.
All of these should be considered for future study.
Conclusion
In this study, though commensals were predominantly isolated, few pathogenic organisms like Staphylococcus aureus, Pseudomonas, Proteus, E.coli and Klebsiella were also isolated from the HCWs making them potential carriers for nosocomial infections. These organisms also exhibited high level of resistance to antibiotics like ampicillin, cotrimoxazole, cefatoxime, ciprofloxacin and amikacin. All the isolates were sensitive to cefepime and cefaperazone-sulbactum combination. Methicillin resistance was also observed among the Staphylococcus isolates.
HCWs are more prone to transmit hospital acquired infections. Physical contact with HCWs is the most frequent mode of transmission of nosocomial infections. Hence awareness should be created among HCWs about the significance of handwashing and use of personal protective equipment to prevent cross-infection. Strict adherence to hand washing and good personal hygiene by the HCWs is the need of hour to prevent health care associated infections.
[1]. Chiller K, Selkin BA, Murakawa GJ, Skin microflora and bacterial infections of the skinJournal of Investigative Dermatology Symposium Proceedings 2001 6(3):170-74.10.1046/j.0022-202x.2001.00043.x11924823 [Google Scholar] [CrossRef] [PubMed]
[2]. Fredricks DN, Microbial ecology of human skin in health and diseaseJournal of Investigative Dermatology Symposium Proceedings 2001 6(3):167-69.10.1046/j.0022-202x.2001.00039.x11924822 [Google Scholar] [CrossRef] [PubMed]
[3]. Marples MJ, Life on Human SkinSci Am 1969 220(1):108-15.10.1038/scientificamerican0169-1085761729 [Google Scholar] [CrossRef] [PubMed]
[4]. Roth RR, James WD, Microbial ecology of the skinAnnu Rev Microbiol 1988 42:441-64.10.1146/annurev.mi.42.100188.0023013144238 [Google Scholar] [CrossRef] [PubMed]
[5]. Cogen AL, Nizet V, Gallo RL, Skin microbiota: a source of disease or defence?Br J Dermatol 2008 158(3):442-55.10.1111/j.1365-2133.2008.08437.x18275522 [Google Scholar] [CrossRef] [PubMed]
[6]. Musu M, Lai A, Mereu NM, Galletta M, Campagna M, Tidore M, Assessing hand hygiene compliance among healthcare workers in six Intensive Care UnitsJ Prev Med Hyg 2017 58(3):E231-37. [Google Scholar]
[7]. Noble WC, Skin microbiology: coming of ageJ Med Microbiol 1984 17(1):1-12.10.1099/00222615-17-1-16229637 [Google Scholar] [CrossRef] [PubMed]
[8]. Roth RR, James WD, Microbiology of the skin: resident flora, ecology, infectionJ Am Acad Dermatol 1989 20(3):367-90.10.1016/S0190-9622(89)70048-7 [Google Scholar] [CrossRef]
[9]. Suchitra JB, Lakshmi Devi N, Impact of education on knowledge, attitudes and practices among various categories of health care workers on nosocomial infectionsIndian Journal of Medical Microbiology 2007 25(3):181-87.10.4103/0255-0857.34757 [Google Scholar] [CrossRef]
[10]. Collins F, Hampton S, Hand washing and methicillin resistant Staphylococcus aureusBr J Nurs 2005 14(13):703-07.10.12968/bjon.2005.14.13.1845116116370 [Google Scholar] [CrossRef] [PubMed]
[11]. Kampf G, Kramer A, Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubsClin Micrbiol Rev 2004 17(4):863-93.10.1128/CMR.17.4.863-893.200415489352 [Google Scholar] [CrossRef] [PubMed]
[12]. Miles RS, Amyes SGB, Laboratory control of antimicrobial therapyMackie & McCartney Practical Medical Microbiology 2007 14th ed:151-78. [Google Scholar]
[13]. Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing M 100-2016; S26th ed. Wayne, PA. CLSI [Google Scholar]
[14]. Chander J, Fungal reagents and stainingTextbook of Medical Mycology 2009 Third edition:514-21. [Google Scholar]
[15]. Sarfraz A, Bhattacharyya S, Ansari MAA, Jaiswal NK, Roy H, Kumar R, Study of bacterial flora of hands of health care givers in a tertiary care hospital in eastern IndiaJ of Evolution of Med and Dent Sci 2015 4(27):4644-48.10.14260/jemds/2015/672 [Google Scholar] [CrossRef]
[16]. Khodavaisy S, Nabili M, Davari B, Vahedi M, Evaluation of bacterial and fungal contamination in the health care workers hands and rings in the intensive care unitJ Prev Med Hyg 2011 52:215-18. [Google Scholar]
[17]. Brunetti L, Santoro E, De Caro F, Cavallo P, Boccia G, Capunzo M, Surveillance of nosocomial infections: a preliminary study on hand hygiene compliance of healthcare workersJ Prev Med Hyg 2006 47(2):64-68. [Google Scholar]
[18]. Kokate S, Rahangdale V, Telharkar P, Nirmal P, Bacterial hand contamination in medical students-a possible carrier of nosocomial infectionsIOSR Journal of Dental and Medical Sciences 2015 14(4):54-56. [Google Scholar]
[19]. Kampf G, Loffler H, Gastmeier P, Hand hygiene for the prevention of nosocomial infectionsDtsch Arztebl Int 2009 106(40):649-55.10.3238/arztebl.2009.064919890431 [Google Scholar] [CrossRef] [PubMed]
[20]. Al-Busaidi S, Health care workers and hand hygiene practice: a literature reviewDiffusion: the UCLan Journal of Undergraduate Research 2013 6(1):1-13. [Google Scholar]