Ovarian carcinoma is one of the most common malignancies of the female genital tract standing next only to cervical cancer [1,2]. Incidence of ovarian cancer is lower in India (3.4/100,000) compared to the West (6.7/100,000) [3]. It usually occurs in the 6th and 7th decades of life [4]. It causes more deaths than any other malignancies of the female reproductive tract [5]. Ovarian malignancies were conventionally treated with primary cytoreductive surgery followed by adjuvant chemotherapy. In 2012, National Comprehensive Cancer Network Guidelines recommended the new treatment approach of neoadjuvant platinum based chemotherapy followed by IDS [6,7]. The aim of this approach, commonly called sandwich therapy is to reduce tumour volume, optimise cyto reduction and improve the chance of complete remission [8]. Neoadjuvant Chemotherapy (NACT) induces numerous morphological changes in the tumour cells and stroma. Some of the morphological changes include nuclear enlargement, nuclear clumping and foamy cell changes. Stromal changes include inflammation, fibrosis, necrosis and dystrophic calcification [9,10]. This study aims to evaluate the histomorphological changes induced by NACT, its influence on the progression free survival of patients with malignant ovarian neoplasms.
Materials and Methods
This retrospective cross-sectional study was conducted in the Pathology Laboratory, VS Hospital, Chennai, during the period of January 2011-December 2016 and consisted of 52 cases treated with NACT followed by IDS. Detailed post-chemotherapy histomorphological changes, particularly the presence of residual tumour, necrosis, fibrosis, inflammation and psammoma bodies were looked for in H&E stained sections representing the entire specimen. The patients were followed up for a period of three years from the date of IDS and time of recurrence of disease or death was noted. Progression free survival denotes the period between IDS and recurrence of disease. The above-mentioned parameters were correlated with survival. Though there are studies stating that grading, staging and subtyping of tumour is not possible after NACT, an attempt has been made in this study to grade and stage the tumour. This study was carried out in collaboration with medical and surgical oncology departments. The follow-up status of some patients was retrieved from the medical records department of VS Hospital. The study was carried out after getting approval from the Institutional Ethics Committee (No.18112011).
Patients having complex adnexal mass, raised CA-125 levels and positive ascitic fluid cytology were included in this study. Patients with benign neoplasms of the ovary and patients who lost follow-up were excluded from the study. Patients having any one or combination of the above-mentioned inclusion criteria were chosen for NACT with carboplatin and paclitaxel, followed by IDS.
Histomorphological changes induced by NACT were evaluated in detail on H&E stained sections. This includes subtyping of tumour, presence of fibrosis, necrosis, residual tumour, inflammation, psammoma bodies, foamy histiocytes, cholesterol clefts, haemosiderin laden macrophages, calcification, foreign body giant cells, tumour giant cells, mitotic figures/10 HPF, surface involvement, grade, stage of the tumour, lymph node metastasis and omental deposits.
Five parameters were graded. Fibrosis was scored as mild (1+), moderate (2+) and severe (3+). Necrosis was graded as absent (0), 1-50% (1+), >50% (2+). Presence of residual tumour was graded as <5% (1+), 5-50% (2+) and >50% (3+). Inflammation was scored as mild (1+) and extensive (2+); psammoma bodies as absent (0) and present (1+). These grading schemes were done on H&E stained sections and grading cut-offs were chosen from previous studies.
The patients were followed up for three years from the date of IDS. The date of recurrence of disease or death of the patient was recorded. The time interval between IDS and recurrence of disease is termed progression free survival. The five parameters which were graded were compared with the survival period and their significance over the survival time was estimated.
Statistical Analysis
The statistical analysis was performed using IBM Statistical Package for Social Sciences Software version 20. Pearson Chi-square test and Kaplan-Meier survival analysis were used to analyse the histopathological parameters and to calculate the progression free survival respectively.
Results
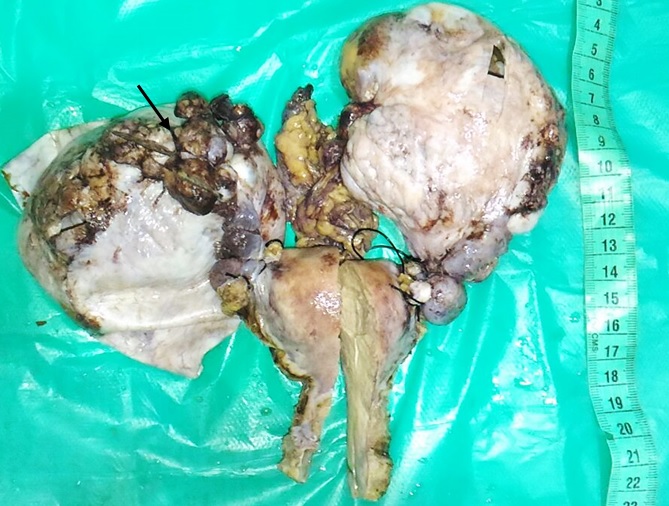
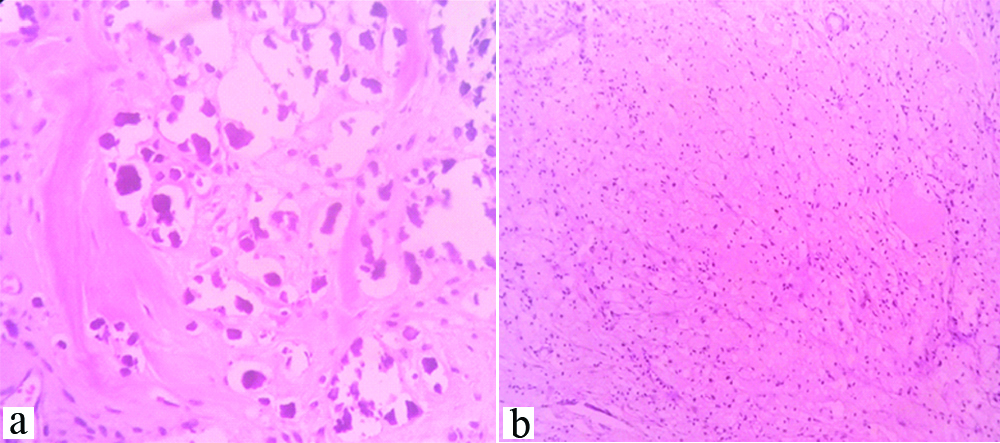
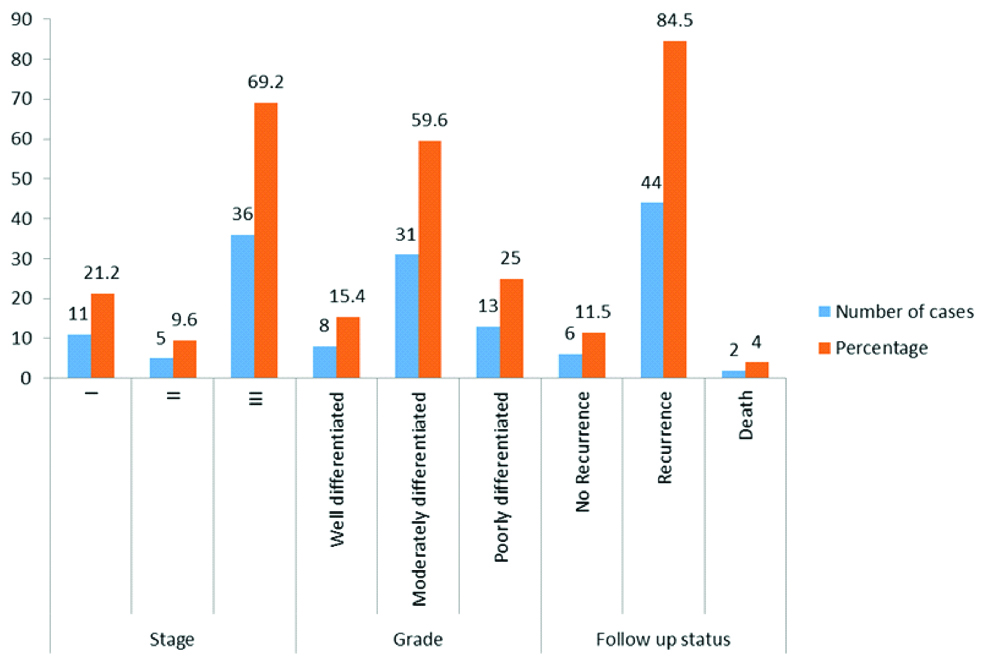
The age of the patients ranged from 34 to 80 years with a mean age of 52 years. Out of the 52 cases, 38 cases (73.1%) had surface involvement [Table/Fig-1,2]. On microscopic examination, 46 (88%) cases were serous cystadenocarcinoma, 4 (8%) were mucinous cystadenocarcinoma, 1(4%) (Case each of endometrioid adenocarcinoma and malignant mixed mullerian tumour. Thirty one cases (59.6%) had moderately differentiated tumour. About 35 cases (67.3%) had 1+fibrosis, 30 cases (57.5%) had 2+residual tumour [Table/Fig-3a], 22 cases (42.3%) had haemosiderin laden macrophages [Table/Fig-3b], 16 cases (30.8%) had 1+inflammation [Table/Fig-4a], residual tumour cells exhibited nucleomegaly and prominent nucleoli [Table/Fig-4b].
Shows hysterectomy and bilateral salpingo-oophorectomy specimen with enlarged ovaries showing surface involvement (black arrow).
Shows cut surface of ovary exhibiting solid and cystic areas, focal yellowish, necrotic and haemorrhagic areas.

(a) shows residual tumour cells arranged as glands and sheets, H&E, 4x; (b) shows sheets of haemosiderin-laden macrophages (red arrow) adjacent to infiltrating tumour cells, H&E, 10x.

(a) shows sheets of chronic inflammatory cells amidst residual tumour cells(red arrow) H&E, 4x; (b) shows residual tumour cells exhibiting nucleomegaly and having prominent nucleoli, H&E,40x.

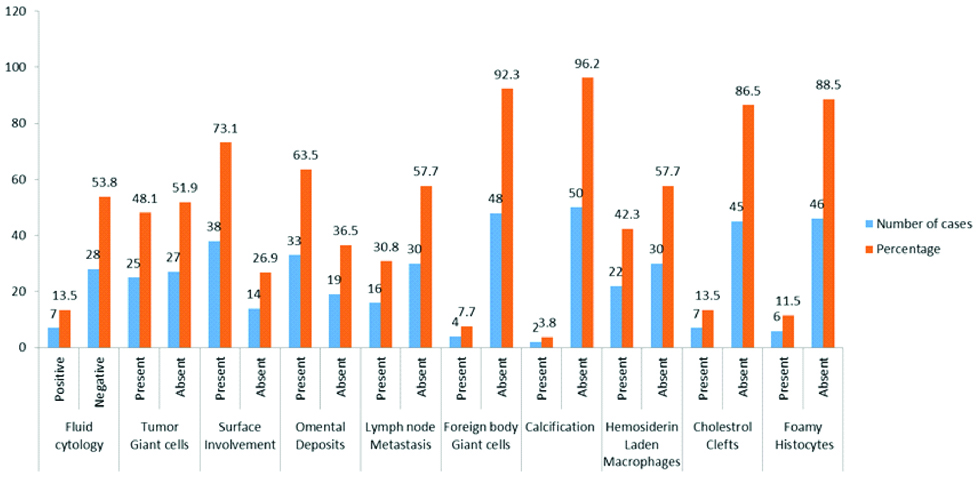
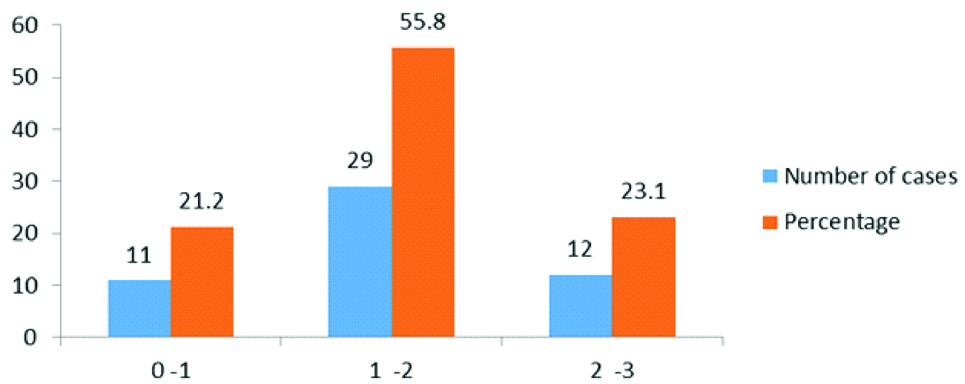
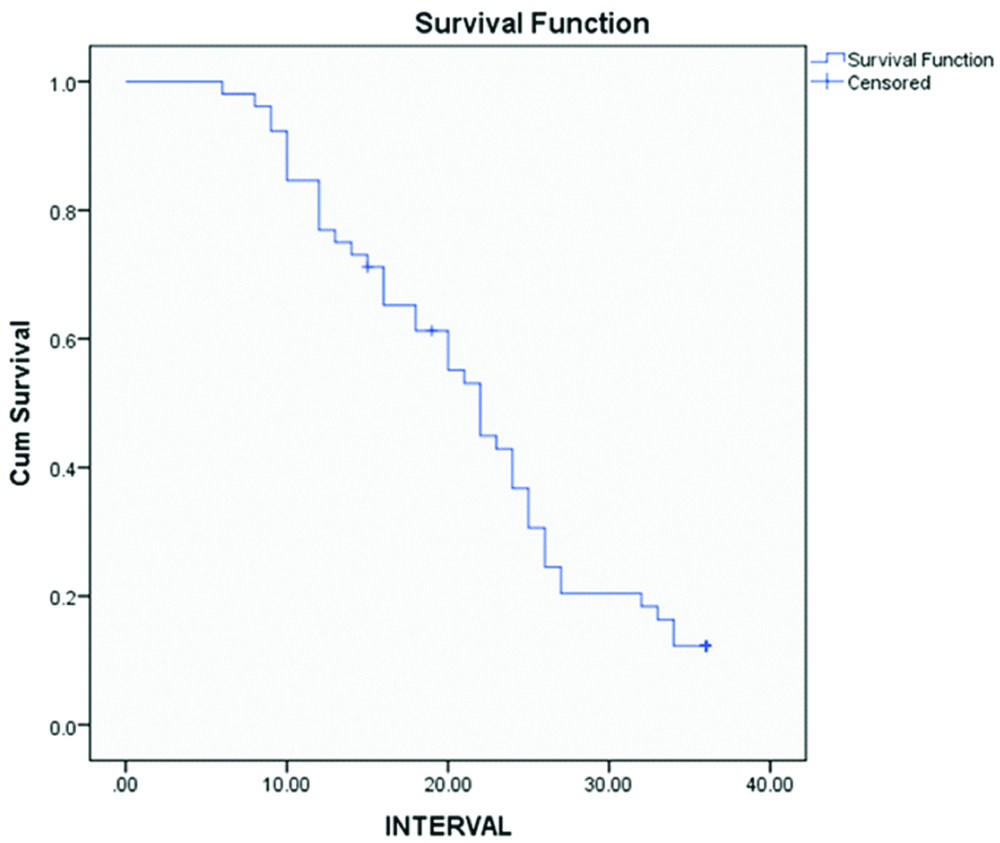
Seven cases (13.5%) had cholesterol clefts and foreign-body type of giant cells [Table/Fig-5a,b], 27 cases (51.9%) had psammoma bodies and foci of calcification [Table/Fig-6a], 6 cases (11.5%) had foamy histocytes [Table/Fig-6b], residual tumour cells exhibited cytoplasmic eosinophilia and apoptotic bodies [Table/Fig-7a] and 12 cases (23.1%) had 2+necrosis [Table/Fig-7b]. Scoring of various parameters and their correlation with progression-free survival is depicted in [Table/Fig-8]. The proportion of various histomorphological changes observed in interval debulking specimens is illustrated in [Table/Fig-9]. Grading of the number of mitotic figures in the residual tumour is depicted in [Table/Fig-10]. The status of patients after three years follow-up from the date of IDS is shown in [Table/Fig-11]. Progression free survival was calculated by using Kaplan-Meier survival analysis as shown in [Table/Fig-12]. The median progression free survival of the patients was 22 months. Among the graded histopathological parameters, presence of necrosis was found to have a statistically significant association with improved progression free survival of the patient (p-value of 0.021).
(a) and (b) shows cholesterol clefts (red arrow) and scattered foreign-body type of giant cells (green arrow), H&E, 10x.

(a) Shows specks of dystrophic calcification, H&E, 40x; (b) shows sheets of foamy histiocytes, H&E,10x.
(a) shows residual tumour cells exhibiting cytoplasmic eosinophilia and nucleomegaly, H&E,40x inset shows an apoptotic cell; (b) shows areas of necrosis, H&E, 4x.

Shows scoring of various parameters and their correlation with progression free survival (Chi-square test).
| Parameters | Scores | Number of cases | Percentage | Number of Recurrences and Death | p-value |
|---|
| Fibrosis | Nil | 3 | 5.7 | 2 | 0.349 |
| 1+ | 35 | 67.3 | 30 |
| 2+ | 7 | 13.5 | 7 |
| 3+ | 7 | 13.5 | 7 |
| Necrosis | Nil | 20 | 38.5 | 20 | 0.021 |
| 1+ | 20 | 38.5 | 18 |
| 2+ | 12 | 23.1 | 8 |
| Residual tumour | 1+ | 6 | 11.5 | 4 | 0.094 |
| 2+ | 30 | 57.5 | 26 |
| 3+ | 16 | 30.8 | 16 |
| Inflammation | Nil | 23 | 44.2 | 20 | 0.254 |
| 1+ | 16 | 30.8 | 13 |
| 2+ | 13 | 25 | 13 |
| Psammoma Bodies | Absent | 25 | 48.1 | 21 | 0.329 |
| Present | 27 | 51.9 | 25 |
Shows the proportion of various histomorphological changes assessed in the interval debulking surgery specimens.
Shows the grading of mitotic figures in the residual tumour.
Shows proportion of parameters like grade and stage of the tumours and three year follow-up status of patients.
Shows estimation of progression free survival using Kaplan-Meier survival analysis.
Discussion
Few studies state that pre-operative biopsy is not a mandatory criterion to start therapy in ovarian tumours and imaging findings of a complex ovarian mass with elevated CA 125 levels with malignant cytology in ascitic fluid were considered sufficient evidence to start definitive therapy [7]. In our study, the above-mentioned criteria were taken into account to start preoperative chemotherapy and only a few cases had preoperative tissue biopsy findings. As grading and staging of the tumour play a very important role in predicting the prognosis, an attempt has been made in this study to grade and stage the tumour post-chemotherapy. However, accurate tumour typing and grading is not possible on tissue samples studied after chemotherapy and there is mounting evidence that grading of ovarian tumours into low and high grade based on morphology is imperative for proper decision making regarding the choice of chemotherapy and to predict the prognosis. Hence, in cases where NACT is planned, a histological tissue biopsy should be obtained if possible, both to confirm the presence of ovarian cancer and to ascertain the morphological subtype.
NACT is an upcoming treatment approach for ovarian malignancies [11]. Various histomorphological changes are induced by NACT and there is definitive need for the reporting pathologists to understand and assess these histopathological changes. Though many studies have documented morphological changes induced by chemotherapy in cancers of colon, breast and oesophagus [12-14], the scenario in ovarian malignancies is different. There are not many studies that have analysed the histopathological changes induced by NACT in ovarian malignancies.
A study done by Samrao D et al., in 67 patients, objectively evaluated these morphological parameters [8]. According to the study done by Mc. Cluggage WG et al., in 18 patients, presence of psammoma bodies without residual tumour was considered good response to chemotherapy [15]. Sassen S et al., were the first to describe the terms morphological responders to NACT in the management of carcinoma ovary [16]. Their study showed that features like presence of scattered tumour cells, fibrosis, foamy macrophages, foreign body giant cells, psammoma bodies, inflammatory cell infiltrates, haemosiderin laden macrophages were present more significantly in the morphological responders. Among these histological parameters evaluated, we aimed at evaluating the findings associated with progression free survival.
In our study, detailed evaluation of histopathological changes induced by NACT was scored and evaluated among the morphological responders. On comparing and extrapolating features like presence of fibrosis, necrosis, inflammation, residual tumour and psammoma bodies against progression free survival period, necrosis was found to have statistically significant association with survival.
The patients were followed up for a period of 3 years from IDS. Time of recurrence of disease was noted. The mean progression free survival estimated by Kaplan Meier survival test was 22 months, which is better when compared to patients who underwent upfront surgery for ovarian malignancies, according to the literature. Also, this study indicates that there is not a very big difference in overall survival of the patients on giving NACT for ovarian cancer and this fact too is supported by literature [17-20].
Limitation
The limitation of this study is the absence of a control group of patients who underwent primary cytoreductive surgery to be compared with patients who get neoadjuvant platinum-based chemotherapy, was overcome by comparing the statistical analysis and the values obtained thereon with the current and existing literature. Patients were followed up only for a period of three years to calculate the progression free survival.
Conclusion
Our study highlights the importance of preoperative biopsy and grading of ovarian tumours, post-neoadjuvant therapy assessment of histomorphological response in resected surgical specimens emphasising the importance of tumour necrosis with progression free survival of patients. The present study also highlights the improvement in progression free survival of patients following neoadjuvant therapy though the overall survival remains the same on follow-up of the patients. Linking morphology to molecular diagnostics, an attempt to study the tissue molecular biomarkers capable of predicting better prognosis and an increase in survival will carry the advances in the field of ovarian cancer to greater heights and carries a vast prospect.