In High Dose Rate (HDR) brachytherapy, applicators are identified using either 2Dimensional (2D) orthogonal radiograph or Computed Tomography (CT) images. Treatment plans are optimised by altering the dwell times with respect to the anatomical point or volume subjected to the normal tissue constraints. These treatment plans are verified before delivery which was not of common practice worldwide [1]. However, verification of planned dose distribution pose a thought provoking task due to nature of high range doses, steep dose gradients and small spatial distributions [2]. Therefore, need of the hour is to find a method to verify dose distribution which was a common practice in external beam radiotherapy [3]. The commercially available Treatment Planning Systems (TPS) employ dose calculation algorithms which do not take into account heterogeneities present in the patient and the applicators shielding effects used in the treatment. This suggests a mismatch between the planned and real delivered dose to the tumour and organs at risk [4]. A number of experimental and Monte Carlo (MC) studies have been reported in literature representing the influence of inhomogeneity in brachytherapy treatments [5-8] and the shielding effect created by the applicators [9,10].
Brachytherapy treatments are currently undergoing a period of significant innovation and rapid transformation, together with a fundamental shift away from the use of traditional pre-determined dose distributions and alteration from 2Dimensional (2D) to 3Dimensional (3D) viewpoints of the entire treatment process. It is essential that quality assurance techniques need to be in line with progresses in brachytherapy treatment planning and delivery to ensure an appropriate level of dosimetric accuracy and quality. Modern 3D-based brachytherapy which requires multi-dimension verification measurements of the planned dose with clinical treatment applicator in-situ and the potential of significant patient-specific dose distribution optimisation [11]. Radiochromic film dosimetry is often employed for dose distribution measurement in radiotherapy, with significant advantage compared to other dosimetry methods, including high spatial resolution, low energy dependence, and near water equivalence [12-15] and relative ease of signal readout with a desktop scanner [16,17].
The independent 3D brachytherapy dosimetry audit needs a development of practical measurement and analysis technique [11]. Advancement in brachytherapy procedures has increased the need for three dimensional dosimeters to verify dose calculation algorithms and treatment deliveries. Development of a stable, easy to analyse 3D dosimeter is essential and could result in broader clinical implementation of 3D dosimetry [18]. Hence, the study was carried out to validate the model based dose calculation algorithm.
Materials and Methods
The study was piloted in order to effectively commission the new algorithm for clinical use. The traditional method of testing newer algorithm is to do simulation study on tissue equivalent phantom which was carried out for six months in the medical physics laboratory.
The materials used in this study are:
HDR Brachytherapy unit (Gammamed Plus iX) with AcurosTM BV algorithm,
Titanium applicators viz. ring with tandom set, vaginal mould with partial shielded (Tungsten), stainless steel interstitial needles,
Tissue equivalent material,
RW3 water equivalent slab phantom,
EBT2 Gafchromic film with EPSON 10000XL scanner,
Film QA Pro software for film analysis,
IBM SPSS software 21.0 version for statistical analysis.
Descriptive analytical study was carried out to effectively commission the new algorithm for calculating the brachytherapy dose in clinical use. A 192Ir brachytherapy treatment planning system (TPS), BRACHYVISION™ has been made available along with heterogeneity algorithm through the implementation of a grid-based Boltzmann equation solver, AcurosTM BV algorithm. Gafchromic films were exposed to different known doses and calibrated for the optical density against the standard values. In order to measure the fluence pattern and validate, tissue equivalent phantoms were designed and a stack of Gafchromic films was arranged to measure planar fluence in multiple planes. These fluences were compared against the planned fluence in treatment planning system using Desktop Epson 10000XL scanner and film QA software. Normally distributed continuous data were presented as mean with standard deviation. Paired t-test was used to compare the mean difference between paired data. A p-value of less than 0.05 was considered significant. All data analysis was done using IBM SPSS version 21.
Brachytherapy Applicators and Phantom
Part I: Experimental setup with interstitial metal catheters: Calibration Jig, Poly Methyl Methacrylate (PMMA) 30×30×1 cm designed for consistent and reproducible grooves slot using a simple set-up configuration with RW3TM solid water phantom slabs above and below this acrylic slab. About five Stainless steel catheters, 200 mm length placed in this grooves, 16 numbers of Gafchromic Eriochrome Black T (EBT2) film stacked above the metal catheter.
Part II: Experimental setup for ring applicator fluence verification: The titanium ring and tandem applicator combination set indicated for high dose rate irradiation of the uterus and cervix. This applicator set manufactured from titanium and is compatible with both CT and Magnetic Resonance (MR) imaging systems. This system features a 32 mm diameter ring with a build-up cap of 5 mm used in conjunction with interlocking tandem of 60 mm length. The applicator includes tandem angle 60 degree with rectal retractor. This combination set is completely placed in toughened dental modelling wax in sheets of 187×88×1.5 mm thick each 16 EBT2 film below the rectal retractor in frontal plane as well as in the sagittal plane to verify the frontal and sagittal plane fluence.
Part III: Experimental setup for vaginal mould partially shielded applicator fluence verification: The shielded applicator set was developed to treat cancer of the vagina, vaginal stump or rectum where partial shielding is required. A variety of cylinder diameters are available to create the optimal distance between the source and the patient’s layers of tissue to be treated. A variety of different shielding positions can be achieved by using a 90° or 180° tungsten alloy shielding segment. After insertion of the applicator, marking screws identify the shielded area.
In this setup, shielded cylinder 30 mm diameter made of Plexiglas® with 180° posterior tungsten alloy shield, the applicator probe made of stainless steel 3 mm diameter×320 mm length used. Anterior to the shielded applicator, 14 EBT2 films and posterior to shielded applicator one EBT2 film placed to verify the fluence in the shielded and unshielded area and to validate the plan.
Brachytherapy: Treatment Planning System and Algorithm
An 192Ir brachytherapy Treatment Planning System (TPS) has been made available BRACHYVISION™ v. 10.0, Varian Medical Systems, Inc., Palo Alto, California. Heterogeneity algorithm is realised through the implementation of a grid based Boltzmann equation solver AcurosTM BV v 1.4.0 developed by Transpire, Inc., Gig Harbor, Washington. The Gammamed iX plus unit (Varian Medical Systems, Palo Alto, California, USA) with 192Ir Gammamed HDR plus source was used.
Gafchromic EBT2 Film, Irradiation and Calibration
Gafchromic EBT2 films were designed to match the characteristics of high energy radiation with low energy dependence and relatively low absorbed dose requirements for analysis. EBT film provides a higher level of dose sensitivity and low energy response variations and seems to be ideally suited for radiotherapy application [19]. Standard calibration of 6 Mega Volt (MV) photon beam from the linear accelerator performed to verify the output (absolute dose). Film samples were cut into 20×4 cm2 sizes and irradiated with 6 MV X-beam. Samples were placed at the isocenter of the accelerator, at a Source to Axis Distance (SAD) set up of 100 cm. A 10×10 cm2 field size at the isocenter was used. The films were covered with a 5 cm thick piece of solid water model (RW3). To obtain a calibration curve, the films were exposed perpendicularly to the radiation beam with various known doses. The maximum dose exposed for calibration film was about 30% greater than the highest dose expected for an application film. The film samples were handled in accordance with the recommendations specified in the American Association of Physicists in Medicine (AAPM) Task Group (TG) 55 report [12].
Film Scanning and Analysis
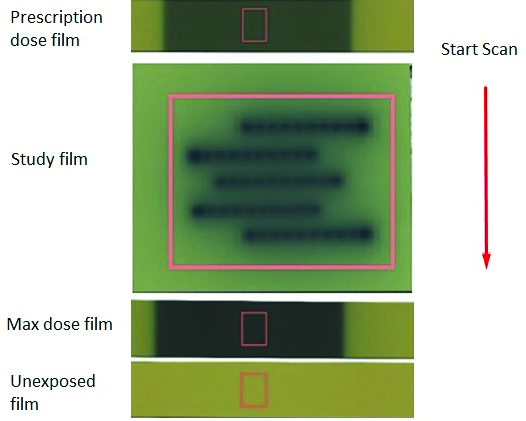
The scanners were fitted with transparency adapters and the images were acquired in transmission mode. The warm up of scanner EPSONTM 10000 XL flatbed Version 3.49A performed for 20 minutes. Films were scanned in portrait orientation set to professional mode, 48 bit Red Green Blue (RGB) mode (16 bit per colour), 72 dpi and saved as tagged image film format (TIFF). The settings were done to deliver the raw data from the scanner without any enrichments. The films were centered on the scan bed and lifted off the glass surface using saturated radiographic films as masks. The EBT2 films were placed 2 cm left of window and at the centre of the transparency adaptor to avoid or reduce lateral response artefact. Positioning of the films on the scan bed was confirmed upto a reproducibility level of one pixel, so the films were compiled and registered without additional transformation to generate a 3D dose distribution [18]. Since radiochromic film is sensitive to the orientation of the film on the scanner, orientation of film in each image was recorded and the same was repeated for the subsequent measurement and analysis [20]. For clinical use and validation of AcurosTM BV algorithm single scan technique is employed which will have unexposed film, maximum dose given to the treatment plan per fraction and application film. Scanned images were measured using film Quality Assurance (QA) Pro software 2015 (Ashland Inc., Wayne, New Jersey). Calibration film strips exposed with 10×10 cm2 fields were measured by defining areas of interest approximately 15.9×13.6 mm2 in size at the centres of the exposed areas. The improved accuracy of the multichannel method comes from its ability to resolve the digital image of a measurement film in two portions, viz., a dose function having image with dose and a disturbance function having image independent of dose and colour [21]. The single scan for multichannel analysis using Gafchromic EBT2 film is shown in the [Table/Fig-1].
EBT2 film scanning orientation in EPSON 10000 XL for multichannel analysis technique.
Gamma Criteria
The original description of gamma criteria, often 3% dose-difference and 3 mm Distance to Agreement (DTA) criteria, respectively. However, based on the clinical relevance this arbitrary value needs to be expanded. According to tumour, normal dose tolerance and dose and spatial uncertainty, the criteria for gamma require modification. As such these criteria is exclusively for patient specific quality assurance in external beam radiotherapy [22,23]. Due to steep dose gradient and dose fall off in brachytherapy along with heterogeneity based dose calculation, various criteria were considered and analysed. The criteria include Dose Difference (DD) of 2% and DTA of 1 mm, DD of 5% and DTA of 1 mm, DD of 10% and DTA of 1 mm. The purpose of the measurement is to validate the output of the treatment planning system, or to validate the dose calculation, dose transfer and dose delivery. TPS calculated doses obtained from different setup were compared with isodoses of actually delivered dose exposed in Gafchromic film and analysed plane by plane.
Statistical Analysis
The data were analysed using paired t-test and the results were tabulated with comparison of mean difference between paired data. International Business Machines (IBM) and Statistical Package for Social Science (SPSS) version 21.0 was used for this entire study and data analysis was carried out. Normally distributed continuous data were presented as mean with standard deviation.
Results
In the part I setup, three different gamma criteria were used to estimate the mean and standard deviation. The entire data inclusive of all criteria used in this study is tabulated [Table/Fig-2]. In the part II setup of the study, both frontal and sagittal plane were evaluated. The comprehensive table both in frontal and sagittal plane EBT2 Gafchromic film analysis were listed in the [Table/Fig-3,4]. The sagittal plane fluence will indicate the dose representing the point A dose and the frontal plane was evaluated below the rectal shield, this will indicate the rectal shield influence on the dose estimated with this heterogeneity algorithm. In frontal plane for gamma pass criteria of 5%/1 mm, the gamma passing rate were 98.96±0.95 as against 93.94±2.45 for 2%/1 mm. Similarly for DTA of 1 mm, 94.21±1.33 was the mean and standard deviation for 2% gamma criteria and 99.49±0.74 for 5% gamma criteria. However, in 10%/1 mm gamma criteria, the dose difference were 99.67%, DTA of 1 mm was 99.91%. The sagittal plane films were analysed 5%/1 mm and 10%/1 mm gamma criteria and results showed above 95% for gamma passing rate.
Interstitial Metal Catheters (IMC) frontal plane fluence verification-average value in different gamma criteria.
| IMC |
|---|
| Gamma Criteria | | N | Mean±SD | Mean Difference | 95% Confidence Interval of the Difference | Sig. (2-tailed) |
|---|
| Lower | Upper |
|---|
| 2%/1 mm | Gamma | 16 | 92.18±1.20 | 92.18 | 91.54 | 92.82 | p<0.001 |
| DD | 16 | 91.84±0.61 | 91.84 | 91.51 | 92.16 | p<0.001 |
| DTA | 16 | 92.11±0.56 | 92.11 | 91.81 | 92.41 | p<0.001 |
| 5%/1 mm | Gamma | 16 | 96.21±1.47 | 96.21 | 95.43 | 96.99 | p<0.001 |
| DD | 16 | 96.41±1.07 | 96.41 | 95.84 | 96.98 | p<0.001 |
| DTA | 16 | 96.73±1.17 | 96.73 | 96.11 | 97.35 | p<0.001 |
| 10%/1 mm | Gamma | 16 | 99.64±0.45 | 99.64 | 99.4 | 99.88 | p<0.001 |
| DD | 16 | 99.52±0.28 | 99.52 | 99.37 | 99.67 | p<0.001 |
| DTA | 16 | 99.84±0.17 | 99.84 | 99.75 | 99.94 | p<0.001 |
R32T60A60 Ring applicator frontal plane fluence verification-average value in different gamma criteria.
| Gamma Criteria | | N | Mean±SD | Mean Difference | 95% Confidence Interval of the Difference | Sig. (2-tailed) |
|---|
| Lower | Upper |
|---|
| 2%/1 mm | Gamma | 16 | 93.94±2.45 | 93.94 | 92.64 | 95.25 | p<0.001 |
| DD | 16 | 92.31±1.36 | 92.31 | 91.59 | 93.04 | p<0.001 |
| DTA | 16 | 94.21±1.33 | 94.21 | 93.5 | 94.92 | p<0.001 |
| 5%/1 mm | Gamma | 16 | 98.96±0.95 | 98.96 | 98.46 | 99.47 | p<0.001 |
| DD | 16 | 96.89±1.48 | 96.89 | 96.1 | 97.68 | p<0.001 |
| DTA | 16 | 99.49±0.74 | 99.49 | 99.09 | 99.88 | p<0.001 |
| 10%/1 mm | Gamma | 16 | 99.93±0.14 | 99.93 | 99.86 | 100 | p<0.001 |
| DD | 16 | 99.67±0.33 | 99.67 | 99.49 | 99.85 | p<0.001 |
| DTA | 16 | 99.91±0.16 | 99.91 | 99.82 | 99.99 | p<0.001 |
R32T60A60 Ring Applicator sagittal plane fluence verification-average value in different gamma criteria
| Gamma Criteria | | N | Mean±SD | Mean Difference | 95% Confidence Interval of the Difference | Sig. (2-tailed) |
|---|
| Lower | Upper |
|---|
| Gamma | 16 | 91.99±0.90 | 91.99 | 91.51 | 92.47 | p<0.001 |
| 2%/1 mm | DD | 16 | 92.57±0.82 | 92.57 | 92.13 | 93.01 | p<0.001 |
| DTA | 16 | 93.73±4.84 | 93.73 | 91.14 | 96.31 | p<0.001 |
| Gamma | 16 | 98.12±1.27 | 98.12 | 97.44 | 98.79 | p<0.001 |
| 5%/1 mm | DD | 16 | 96.60±1.21 | 96.60 | 95.96 | 97.25 | p<0.001 |
| DTA | 16 | 98.21±1.27 | 98.21 | 97.54 | 98.89 | p<0.001 |
| Gamma | 16 | 99.77±0.18 | 99.77 | 99.67 | 99.87 | p<0.001 |
| 10%/1 mm | DD | 16 | 99.60±0.22 | 99.60 | 99.48 | 99.72 | p<0.001 |
| DTA | 16 | 99.66±0.29 | 99.66 | 99.51 | 99.82 | p<0.001 |
The entire analysis was carried out in the plane above unshielded area and in the plane below shielded area. As expected the EBT2 Gafchromic film placed below shielded region showed no exposure or fluence. The analysis in the unshielded region for different criteria was listed in [Table/Fig-5].
Vaginal mould with posterior 180° shielded applicator frontal plane fluence verification in unshielded segment-average value in different gamma criteria.
| Vaginal Mould with posterior shield |
|---|
| Gamma Criteria | | N | Mean±SD | Mean Difference | 95% Confidence Interval of the Difference | Sig. (2-tailed) |
|---|
| Lower | Upper |
|---|
| 2%/1 mm | Gamma | 14 | 91.42±0.56 | 91.42 | 91.09 | 91.74 | p<0.001 |
| DD | 14 | 91.28±0.90 | 91.28 | 90.76 | 91.8 | p<0.001 |
| DTA | 14 | 91.51±0.85 | 91.51 | 91.01 | 91.99 | p<0.001 |
| 5%/1 mm | Gamma | 14 | 97.84±1.93 | 97.84 | 96.73 | 98.95 | p<0.001 |
| DD | 14 | 96.89±1.47 | 96.89 | 96.04 | 97.73 | p<0.001 |
| DTA | 14 | 97.99±1.43 | 97.99 | 97.16 | 98.81 | p<0.001 |
| 10%/1 mm | Gamma | 14 | 99.81±0.24 | 99.81 | 99.67 | 99.94 | p<0.001 |
| DD | 14 | 98.97±1.07 | 98.97 | 98.35 | 99.59 | p<0.001 |
| DTA | 14 | 99.71±0.36 | 99.71 | 99.51 | 99.92 | p<0.001 |
In the part III experimental setup, for the 2%/1 mm gamma criteria, 91% pixels passed in all the three sub divisions of analysis like gamma passing rate, dose difference and DTA. The 5%/1 mm and 10%/1 mm pass criteria indicates 97% and 99% pass rate respectively. The overall significance of paired t-test value is p<0.001. The results of each plane were analysed in all the experimental setup, since each plane is 2D plane verification and stack of all these plane can be considered as 3D verification. Plane by plane comparison were also done and Pearson correlation test were used for plane by plane analysis. Individual evaluation of 2%, 5% and 10% of gamma passing rate, 2%, 5% and 10% of dose difference and 1 mm dose difference on the above criteria were evaluated. The results showed improved passing criteria in AcurosTM BV based dose calculation when compared to TG-43 based calculation.
A 3D Stack Film Analysis
The stacked EBT2 Gafchromic films were analysed plane by plane. In the Interstitial Metal Catheters (IMC) frontal plane analysis, the gamma value for 2% criteria range from 90% to 93.9%, in 5% criteria the value range from 93.8% to 99.8% and in 10% criteria the value range from 98.3% to 100%. When compared plane by plane in Dose Difference (DD) for the same study, under the 2% criteria, the value range varies from 91% to 92.8%, under 5% criteria the value of DD range from 94.1% to 97.8% and under 10% criteria the value of DD range from 99.1% to 100%. The Distance To Agreement (DTA) for all criteria were set to 1 mm only. This is set because the dose variation in brachytherapy is so rapid and for validation of heterogeneity algorithm, it will be more appropriate to use 1 mm DTA unlike external beam radiotherapy plan verification where 3 mm DTA is standard. Under 2%, 5% and 10% criteria the DTA value in IMC setup the range were 91.2% to 93.1%, 94.2% to 99.2% and 99.5% to 100%.
In ring applicator, the acetal ring cap and rectal retractor used along with titanium applicator to validate plan in heterogeneity based dose calculations. The lateral fluence (sagittal) and anterior fluence (frontal) were generated in single exposure simultaneously and analysed. This was done to verify the impact of rectal shield in ring applicator and scatter dose due to this shield as well as in the unshielded area. Seventeen films were stacked in the frontal plane and sixteen planes were stacked in the lateral plane. The 2% gamma criteria under frontal plane range from 88.1% to 96.2% and in sagittal plane the value range from 90.4% to 93.8%. In 5% gamma criteria, the frontal plane value range from 97.3% to 100% and in sagittal plane the significance range from 96% to 100% whereas in 10% gamma criteria the value range from 99.5% to 100% in frontal plane and 99.4% to 100% in sagittal plane.
The Dose Difference in frontal plane under 2% criteria ranges from 90.1% to 94.3% in frontal plane and 91.3% to 94.4% in sagittal plane. In 5% DD criteria, the value of frontal and sagittal plane ranges from 93.5% to 98.5% and 94.6% to 99.8%. Similarly under 10% DD criteria, frontal plane significance range from 99.4% to 100% and 99.3% to 99.9% for sagittal plane. The Distance To Agreement were kept as 1 mm for all the criteria’s say 2%, 5% and 10%. The frontal and sagittal plane under 2% for DTA of 1 mm range from 92% to 96.3% and 89% to 97.3%. In 5% criteria for DTA of 1 mm the value ranges from 97.7% to 100% and 96.3% to 100% in frontal and sagittal plane. Similarly under 10% criteria for the DTA of 1 mm the value range from 99.4% to 100% and 99.3% to 100%. All the value showed here in the study are acceptable for stringent constraints.
The purpose of third study is to evaluate whether the dose was delivered in the shielded part of the segment and to verify that the scatter dose add up a dose in the shielded area in the vaginal mould with posterior shielded applicator. In this, stack film were placed both in the shielded as well as in the unshielded area. The shielded area showed no dose to compare and appeared to have dose contributed due to film scanned along with the setup arrangement for 3D image acquisition in computed tomography scanner. The same was confirmed with TPS calculation under heterogeneity algorithm. The unshielded portion of the applicator where films are stacked showed dose and were analysed with different gamma criteria 2%, 5% and 10%. In the 2% gamma criteria the value of pass percentage range from 90.4% to 92.2%, in 5% criteria pass percentage value range from 95.2% to 100% and similarly in 10% criteria the pass percentage value range from 99.3% to 100%. The Dose Difference under 2%, 5% and 10% showed value range from 90% to 92.7%, 95% to 99.6% and 97% to 100% respectively. The Distance To Agreement were kept as 1 mm in all the three criteria (2%, 5% and 10%) and the pass percentage value range from 90% to 92.7%, 96.1 to 100% and 98.9 to 100%.
Discussion
Only very few literatures comprehensively reviewed recent developments in TPS algorithms in brachytherapy. New model-based dose calculation algorithms are being familiarised that will fundamentally change the complexity and potentially accuracy of treatment planning in brachytherapy. In moving from the traditional TG-43 formalism, the limitations of liquid water dosimetry and dose distributions due to scattering conditions, enabling dose distributions in the presence of heterogeneities and patient scatter conditions be assessed with improved accuracy [24].
AcurosTM BV calculates dose distributions through solving the Linear Boltzmann Transport Equation (LBTE). The AcurosTM BV algorithm was developed to provide accurate and rapid dose calculation for high dose rate (HDR) brachytherapy treatments. In HDR brachytherapy, heterogeneities introduced by finite patient dimensions, anatomical material differences, and applicator materials may all significantly influence the patient dose distribution. Quantitative results and experimental verification of TG-43 and AcurosTM BV algorithms were validated [25]. All code implementations of Monte Carlo (MC), Collapsed Cone (CC), or GBBS methods as MBDCA dose engines involve compromises between computational speed and sufficient dose calculation accuracy and, therefore, the resulting solutions will have a certain amount of uncertainty. These code implementations must be carefully benchmarked against MC or experimentally when uncertainties adequately permit to ensure sufficiently accurate dose prediction within the intended domain [26].
The dose distributions of a HDR 192Ir source in a homogeneous water geometry was reported using BrachyVision TPS (BrachyVision v 8.8, Varian Medical Systems, Palo Alto, CA) with the Model Based Dose Calculation (MBDCA) based AcurosTM system [27]. The percent dose difference with Monte-Carlo N-Particle Extended Code (MCNPX) simulations of the model VS2000 HDR192Ir source centred in a spherical water phantom of radius R=15 cm, and output from a TG-43-based TPS using vendor supplied F(r, θ) tables. The dose differences were large near the cable; the impact on clinical outcomes was negligible with dose differences of less than 3% reported. These results are found to be in good agreement with the results of the work done. Based on the recommendations given in International Atomic Energy Agency Technical Report Series 430 report [28], the commissioning of brachytherapy treatment planning systems, a 5% dose/2 mm distance criterion for gamma function was studied and the results showed 95% passing rate [13]. Whereas in this study for the gamma pass criteria of 2% and 1 mm, 92% were passed, in 5% and 1 mm criteria 96% passed on average and with 10% and 1 mm criteria, almost 100% passing rate were achieved. In reviewing the literature, the common standard set for gamma criteria in brachytherapy is 3% and 3 mm [29] but currently in brachytherapy 5% and 2 mm gamma criteria were followed. Fairly small number of researchers has used tight constraints like 2% and 2 mm criteria for evaluation. Since the brachytherapy dose distribution involved more heterogeneity, the dose will be very high near the source and the dose fall-off will be so rapid. To verify this dose with heterogeneity algorithm with gamma pass criteria of 5% and 1 mm shown a gamma pass rate of 96%, is good enough to prove the acceptable commissioning process.
The protocol adopted for this study is radically not different from customary protocol, but improved. It simply minimises the number of dose points by allowing all the calibration films to fit easily on the scanner together. While this specific improvement is not philosophical, scanning of all EBT2 Gafchromic calibration films at once eliminates effects from inter-scan inconsistency and the decline in the number of dose points reduces the overhead in labour and materials [30]. The differences between results of TPS and film measurements as shown in Lewis M et al., [31] might be due to TPS calculation algorithm in which a uniform water equivalent material is assumed [32]. In this study, the appropriate 3D TPS dose distribution to determine dwell times and positions of 192Ir sources was done and the accuracy of treatment planning using dose distribution map of film dosimeters was evaluated and compared with TPS dose calculations.
The γ tool has proven useful in the quantitative evaluation of dose distributions. Most of the dose measurement and evaluation software has incorporated γ into the evaluation tool suite. The sensitivity of the dose difference tool to steep dose gradients led to advance of the distance-to-agreement [33,34]. The original description of γ used constant criteria, often 3% and 3 mm dose-difference and DTA criteria, respectively. However, this is entirely arbitrary and should be expanded to consider clinical relevance. For example, the dose-difference at low doses could exceed pass criteria with no clinical consequence, or other parameters like distance agreement could be considered more important near a critical structure than in the periphery of the dose distribution. The criteria should be modified according to clinical needs, and ideally according to prescribed tumour dose and normal organ dose tolerances and spatial uncertainty. In this study three different criteria used such as 2%/1 mm, 5%/1 mm, 10%/1 mm for comparison and analysed plane by plane. The grid (Pixel) used for dose calculation in the treatment planning system using heterogeneous algorithm and pixel used for Gafchromic EBT2 film fluence verification were same. Considering all factors discussed, like EBT2 Gafchromic film, film scanning technique, software tool, criteria for validation, stack technique for heterogeneity algorithm, the results showed good agreement.
Limitation
The limitations in this study are quantitative uncertainties not recommended due to the scarcity of the literature on this topic and results based on different possibilities of applicator and or site. Based on available choices within the TPS, accountability for some of these may fall to the medical physics fraternity. There is a notable limitation on the applicator supported by this heterogeneity algorithm with non-changeable CT Hounsfield Unit (HU) to material and density conversions. The patient geometry be applied as per the user need and also should define boundary limits on dose calculation grid size. Currently the Grid Based Boltzmann Solver (AcurosTM BV) algorithm is used for computing the dose due to heterogeneity and the cons in TG-43 however; it does not apply for optimisation. Speedy optimisation technique to correct for heterogeneity and scatter to be incorporated in the future.
Conclusion
The core issue of how heterogeneity dose calculation accuracy was validated using stack of EBT2 Gafchromic film with 192Ir HDR source were described. The Grid Based Boltzmann Solver, MBDCA algorithm were commissioned in treatment planning system with dose measured and validated against golden standard Monte Carlo data (Published). These results agreed within 2% uncertainty. The latest technique of scanning of film with single scan protocol of standard dose along with the exposed plan showed improved method. On the other hand for patient specific dose calculation, it is significant to derive the accurate tissue material from CT Hounsfield units (material density information).
Incorporating correct material density information paves a way for accurate dose prediction using heterogeneity algorithm. The use of film still proved to be a golden standard in clinical audit and commissioning purpose with significant advantage of self-development. Film measurements in multiple planes around an HDR treatment applicator have shown acceptable agreement with TPS with gamma criteria of 5%/1 mm and 10%/1 mm. EBT Gafchromic film can be used for independent quality audit. All the methods provide a comprehensive verification for commissioning the MBDCA-AcurosTM BV and are useful 3D dosimetric tool for Quality Assurance (QA) program in HDR brachytherapy.