Swyer Syndrome in Phenotypic Female with 46,XY Karyotype
D Prashanth Shetty1, Meenakshi Arumugam2, Jayarama S Kadandale3, Suchetha Kumari4
1 Professor and Coordinator, Department of KSHEMA Centre for Genetic Services, K.S. Hegde Medical Academy, Mangaluru, Karnataka, India.
2 Lecturer, Department of KSHEMA Centre for Genetic Services, K.S. Hegde Medical Academy, Mangaluru, Karnataka, India.
3 Adjunct Professor, Department of Cytogenetics, Centre for Human Genetics, Bengaluru, Karnataka, India.
4 Professor, Department of KSHEMA Centre for Genetic Services, K.S. Hegde Medical Academy, Mangaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Mrs. Meenakshi Arumugam, Lecturer, Department of KSHEMA Centre for Genetic Services, K.S. Hegde Medical Academy, Mangaluru-575018, Karnataka, India.
E-mail: hellomeenaa@gmail.com
Individuals with Swyer syndrome have an XY karyotype and are phenotypically female. The prevalence of Swyer Syndrome is about 1 in 30,000 and it is an extremely rare condition. The present study aimed to describe the phenotypic and genotypic variations of two patients with Swyer syndrome. Case 1: A 24-year-old female who presented with primary amenorrhea, infertility with increased FSH level (59.91 mLU/mL) and Case 2: A 29-year-old female with primary infertility was referred. A 2 mL of peripheral blood was drawn for karyotyping. Cytogenetic analysis was carried out using standard GTG banding technique. Cytogenetic analysis revealed a 46,XY karyotype in Case 1 and 46,XY,15cenh+ in Case 2. Q-Banding confirmed the presence of Y chromosome. In addition, Fluorescence In Situ Hybridization (FISH) using CEP X and Y probe and LSI SRY/CEPX probe confirmed the presence of SRY on the Y chromosome. The result showed the presence of sex-determining region of the Y chromosome and also validating the cytogenetic and molecular cytogenetic interpretations. Earlier diagnosis is important and karyotyping is mandatory in these cases.
Cytogenetics, Fluorescence in situ hybridization, Karyotyping, Primary amenorrhea, Primary infertility
Case Report
Case 1
A 25-year-old female was presented for primary amenorrhea and infertility. She is the second child of non-consanguineous parents. She has one younger brother and there was no family history of menstrual problem. She was apparently normal until 14-years of age, attained menstruation only after taking Oral Contraceptive Pills (OCP) as prescribed by the doctor. She got married at the age of 22-years. On general examination, she was 176 cm tall and weighed 90 kg. Her Body Mass Index (BMI) was 29.05 Metric Units. There was no history of diabetes mellitus, tuberculosis, thyroid dysfunction and epilepsy. There was no other case with a history of infertility in the family.
Patient had development of breast from 15-years of age, developed pubic hair from the age of 13-years. Her phenotype was completely normal female and was well built and nourished. Examination of secondary sexual characteristics revealed that the breast was in Tanner’s stage 2 and discharge present from nipple on expression. She had presented with hirsutism also. Her cervix and vagina were healthy. Ultrasonography of the pelvis revealed a hypoplastic uterus and ovaries could not be detected. Her FSH level was 59.91 mIU/mL (Normal range: 2.6-11 mLU/mL) [1].
Case 2
A 29-year-old female was presented for karyotyping analysis with a complaint of Primary Infertility with a bicornuate uterus. She used to take OCPs once in two to three months and menstruates on taking cyclical combined oral contraceptive pills. The phenotypic evaluation showed breast with Tanner stage 3, Pubic and axillary hair were present, the cervix and vagina were healthy. She had Hypogonadotropic Hypogonadism (Secondary Amenorrhoea). Ultrasound examination showed hypoplastic uterus with non-visualisation of ovaries and streak gonads.
She was the second child of non-consanguineous parents. She has one elder sister and one younger sister, both got married and have normal children. She got married to a 38-year-old man four years back and the couple had no issues for all those years, therefore they approached the doctor. On general examination, she was 163 cm tall and weighed 52 kg. Her BMI was 19.6 Metric Units. There was no history of diabetes mellitus, tuberculosis, thyroid dysfunction and epilepsy.
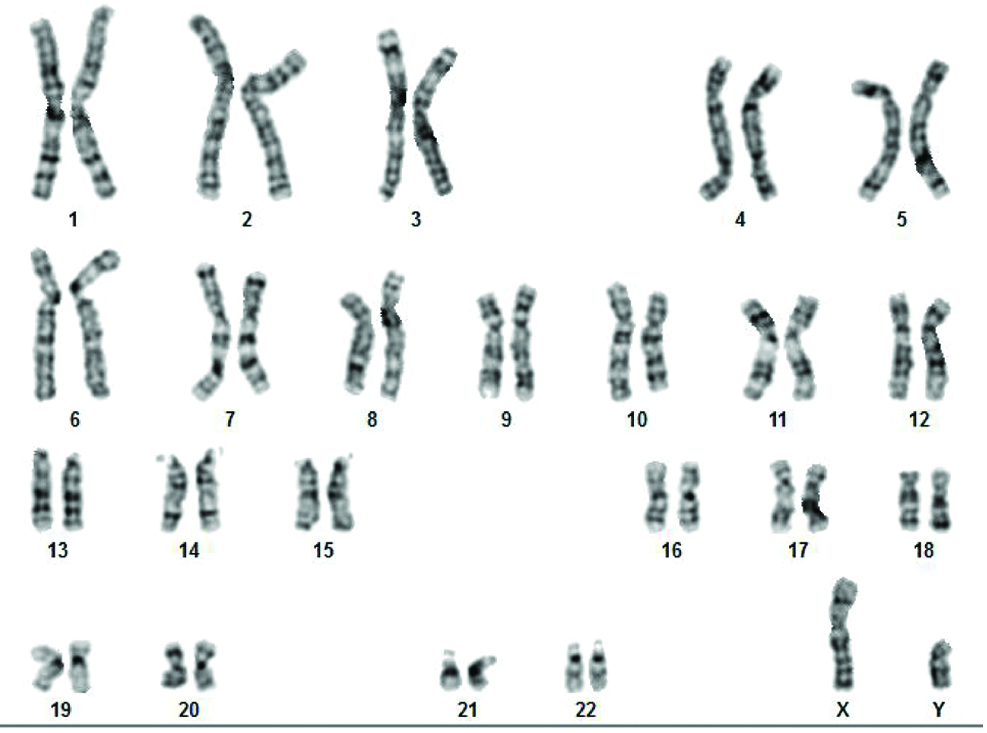
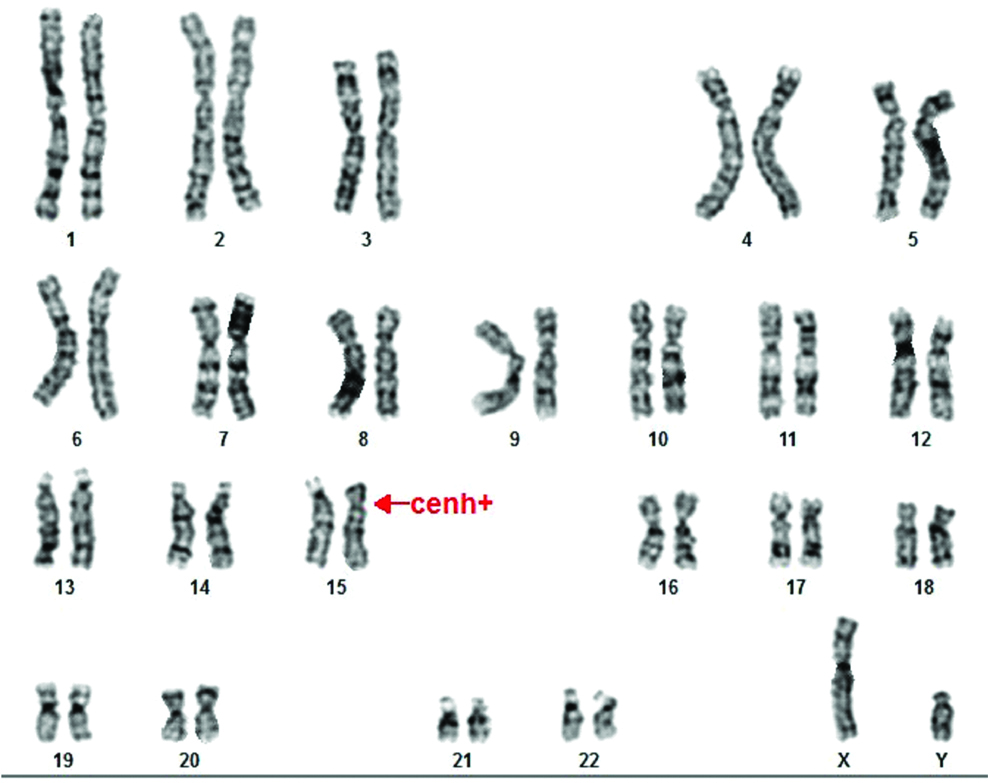
This study was approved by Institutional Ethics committee and informed consent forms were received from patients. A 2 mL of peripheral blood in sodium heparin vacutainer was collected for Chromosomal analysis. Standard GTG banding (G-banding with Trypsin-Giemsa) protocol was carried out. Twenty well-spread metaphases were analysed using bright field microscope [2]. The karyotype of Case 1 was 46,XY [Table/Fig-1] and in Case 2 46,XY,15cenh+ [Table/Fig-2]. Special Quinacrine stain [Table/Fig-3] was performed to confirm the Y chromosome.
Female karyotype with 46,XY.
Female karyotype with 46,XY,15cenh+.
Quinacrine staining of metaphase showing the presence of Y chromosome.

FISH on interphase nuclei using CEP 18;X;Y probe (Cytocell, UK) showed the presence of only XY cell lines (100%) in both the cases [Table/Fig-4]. Chromosomal analysis and FISH were performed using Gene ASIS 7.2 software and reported based on an International System for Human Cytogenetic Nomenclature (ISCN) 2013 [2].
Interphase cells showing one green signal for sex chromosome X and one red signal for sex chromosome Y indicate the presence of XY.

FISH using LSI SRY/CEPX probe showed one signal for CEPX (green) on X chromosome and one signal for SRY (red) on Y chromosome [Table/Fig-5]. Genetic counseling was given to these cases and they were informed about the risk of development of malignancy in the dysgenetic gonads.
Metaphase showing one green signal for sex chromosome X and one red signal for SRY on Y chromosome.

Discussion
Since the first description of Swyer syndrome in 1955, very few cases (<100) have been reported in the literature worldwide [3]. Affected females are not diagnosed earlier until their puberty when primary amenorrhea is evident due to lack of oestrogen and progesterone production [4]. Often differentiating feature of women with Swyer syndrome was height, and the median height in literature was 173 cm [3]. In Case 1, she was 176 cm and the tallest among women affected with Swyer syndrome. Height is also helpful in distinguishing patients with 45,XO/46,XY mosaicism but without the classic features of Turners syndrome [5]. Eventually, we checked for the low-level mosaicism by FISH and proved that they were having 100% of 46,XY karyotype.
Gonads are undifferentiated at sixth week of the intrauterine life and gonads show testicular development in the presence of Testis Determining Factor (TDF) gene on Y chromosome [6]. In the present Case 1, the serum Follicle Stimulating Hormone (FSH) level was 59.91 mIU/mL indicating that the ovaries were not functioning and the ultrasound report also revealed streak gonads. Swyer syndrome is a sex reversal disorder caused by Embryonic testicular regression sequence [7]. The aetiology of 46,XY gonadal dysgenesis is thought to be a deletion involving SRY region on Y chromosome or a mutation in other genes leads to inhibit SRY function [8].
The incidence of these mutations or deletion in SRY was 20% [5,6]. The only gene which is definitively known to play a role in the sexual differentiation is SRY gene; however, its mechanism is unknown [6]. Both cases showed the presence of SRY gene by FISH. However, mutations of SRY identification requires molecular studies, we could not able done by FISH. The presence of Y chromosome is associated with the development of gonadoblastomas and other malignancies in dysgenesis gonads [9]. The frequency of occurring tumour development in pure XY gonadal dysgenesis was 20-30% [5,6], dysgerminoma and gonadoblastoma were reported in about 50% of cases [10].
Similar Swyer syndrome cases have been reported [11-13]. We made the diagnosis of Swyer syndrome for both cases because both patients were phenotypically normal, presented with primary amenorrhea, hypoplastic uterus and pure XY genotype. There were no similar cases in their families. Gonadal dysgenesis patients should undergo gonadectomy to prevent the risk of malignant transformation. However, uterus should be left to give future option of pregnancy [6]. Case 1, underwent laparoscopy and both the streak ovaries were removed. She was counselled for future Artificial Reproductive Therapy (ART) with donor eggs.
Conclusion
The patients can lead a normal life and can conceive using donor oocytes by ART with the aid of Hormone Replacement Therapy (HRT). Health professionals should keep in mind about gonadal dysgenesis if the patients presenting with primary amenorrhea. Earlier diagnosis is important and karyotype should be advised.
[1]. Carmina E, Stanezyk FZ, Lobo RA, Laboratory assessment In: strauss JF, Barbieri RL, edsYen and Jaffee’s Reproductive endocrinology 2014 7th edPhiladelphia, PAElsevier SaundersChapter-34 [Google Scholar]
[2]. Shaffer LG, McGowan-Jordan J, Schmid M, An International System for Human Cytogenetic nomenclature 2013 1st edBaselS. Karger Publishers:01-140. [Google Scholar]
[3]. Michala L, Goswami D, Creighton SM, Conway GS, Swyer syndrome: presentation and outcomesBJOG 2008 115:737-41.10.1111/j.1471-0528.2008.01703.x18410658 [Google Scholar] [CrossRef] [PubMed]
[4]. Machado C, Pereira A, Cruz JM, Cadilhe A, Silva A, Pereira A, A novel SRY nonsense mutation in a case of Swyer syndromeJournal of Pediatric and Neonatal Individualized Medicine 2014 3(1):e030107 [Google Scholar]
[5]. Azidah AK, Nik Hazlina NH, Aishah MN, Swyer syndrome in a woman with pure 46, XY gonadal dysgenesis and a hypoplastic uterusMalaysian Family Physician 2013 8(2):58-61. [Google Scholar]
[6]. Kerimoglu OS, Incesu F, Yilmaz SA, Pekin AT, Dogan NU, Celik C, Dysgerminoma complicating Swyer Syndrome: A case reportIJMMS 2013 1(2):15-16. [Google Scholar]
[7]. Bagci G, Bisgin A, Karauzum SB, Trak B, Luleci G, Complete gonadal dysgenesis 46,XY (Swyer syndrome) in two sisters and their mother’s maternal aunt with a female phenotypeFertility and Sterility 2011 95(5):1786e1-e3.10.1016/j.fertnstert.2010.11.03421145048 [Google Scholar] [CrossRef] [PubMed]
[8]. Behtash N, Zarchi MK, Dysgerminoma in three patients with Swyer syndromeWorld Journal of Surgical Oncology 2007 5:7110.1186/1477-7819-5-7117587461 [Google Scholar] [CrossRef] [PubMed]
[9]. Eunice M, Kulshreshtha B, Kriplani A, Dada R, Agarwal S, Kucheria K, Familial pure gonadal dysgenesis with 46, XY karyotype in three siblings and gonadoblastoma in the youngest siblingInt J Hum Genet 2009 9(2):123-26.10.1080/09723757.2009.11886066 [Google Scholar] [CrossRef]
[10]. Han Y, Wang Y, Li Q, Dai S, He A, Wang E, Dysgerminoma in a case of 46, XY pure gonadal dysgenesis (swyer syndrome): a case reportDiagnostic Pathology 2011 6:8410.1186/1746-1596-6-8421929773 [Google Scholar] [CrossRef] [PubMed]
[11]. Babre VM, Bendre K, Niyogi G, A rare case of Swyer’s syndromeInt J Reprod Contracept Obstet Gynecol 2013 2:485-87.10.5455/2320-1770.ijrcog20130955 [Google Scholar] [CrossRef]
[12]. Bhat BPR, Navada HM, XY Female: Two cases with Different Gonads presenting as Primary AmenorrheaWorld J Endocr Surg 2012 4(1):23-25.10.5005/jp-journals-10002-1088 [Google Scholar] [CrossRef]
[13]. Bansal S, Malhotra N, Dhadwal V, Deka D, Mahendru R, Swyer syndrome with gonadoblastoma: a rarity of two case reportsWomen’s Health Gynecol 2015 1:01-02. [Google Scholar]