MMA-based (acrylic) resins are widely used in dentistry for various applications, including fabricating removable orthodontic appliances [1]. These appliances are used for space maintenance, overbite reduction, tipping teeth and post-orthodontic retention. Auto-polymerized MMA-based resins are popular for orthodontic purposes due to their ease of handling [2]. The polymerization of auto-polymerized PMMA can be completed at room temperature within a short time without the use of any additional equipment [3].
Auto-polymerized acrylic resins have advantages over heat-polymerized acrylic resins, in terms of ease of use and rapid curing. However, the degree of conversion is not as high as for heat-polymerized resins [2], thus a larger amount of unreacted monomer can be found in auto-polymerized resins compared with heat-polymerized resins [2,3]. This residual monomer can leach out in the oral cavity and contact the oral mucosa, leading to erythema, necrosis, pain, or burning sensation, however these reactions vary among patients [4-7]. Furthermore, many studies have reported the deleterious effects of residual monomer on the physical properties of acrylic resin [8,9]. Therefore, residual monomer should be reduced to as low a level as possible before inserting the appliance into the mouth [10].
There are many methods to reduce the amount of residual monomer in auto-polymerized acrylic resins, including immersion in warm water [11-13] and microwave irradiation [13-15]. These methods not only reduced residual monomer, but also reduced the cytotoxicity [11,12,14,16,17]. However, these methods require a long time and use some unusual equipment. Thaitammayanon P et al., demonstrated that ultrasonic immersion, which is usually used for cleaning dental instruments, was a short time method to reduce amount of residual monomer in MMA-based orthodontic base-plate materials [18]. Although an ultrasonic bath can quickly reduce the amount of residual monomer, the effect on the physical properties of an MMA-based orthodontic base-plate material has not been reported.
The aim of this study was to investigate the effects of ultrasonic immersion in 50°C water and various concentrations of 50°C ethanol solutions as a post-polymerization treatment in reducing the level of residual monomer. The flexural strength, flexural modulus, microhardness, water sorption, and water solubility of the material was investigated. The null hypothesis of this study was that the physical properties of the treatment groups were not different from those of the control group.
Materials and Methods
This laboratory study was conducted at the Faculty of Dentistry, Chulalongkorn University. The sample size calculation was performed according to a previous study [19].
Sample Preparation
An MMA-based orthodontic base-plate material (Orthoplast, Vertex-Dental, Soesterberg, The Netherlands) [Table/Fig-1], was prepared at a 2.7:1 powder to liquid ratio. Eighty rectangular specimens and 80 disc-shaped specimens were prepared using a spray-on technique in stainless steel moulds. The moulds were placed in a pressure cooker with 250 kPa (2.5 bar) pressure and 55°C temperature for 20 minutes per the manufacturer’s instruction.
Chemical composition of MMA-based orthodontic base-plate materials.
| Brand | Components | Composition | Manufacturer | Batch number |
|---|
| Orthoplast (OP) | Powder | Polymethyl methacrylate >99%, accelerator <1%, color agents <1% | Vertex-Dental, Soesterberg, The Netherlands | B4-957 |
| Liquid | Methyl methacrylate >95%, ethylene glycol dimethacrylate <5% | 14003860 |
The specimens were wet polished with P500, P1000, and P1200 metallographic grinding paper (TOA, Bangkok, Thailand) to the size recommended by ISO 20795-2 [20]. (64 mm×10 mm×3.3 mm for rectangular specimens, and 50 mm diameter×0.5 mm thickness for disc-shaped specimens) and stored at -20°C until used. The specimens of each shape were divided into eight groups (n=10) as shown in [Table/Fig-2] [21].
| Group | Treatment |
|---|
| I | Untreated (control) |
| II | Water immersion 25°C, 24 hours |
| III | Water immersion 25°C, 72 hours |
| IV | Ultrasonic, water 50°C, 10 minutes |
| V | Ultrasonic, 10% ethanol, 50°C, 10 minutes |
| VI | Ultrasonic, 20% ethanol, 50°C, 10 minutes |
| VII | Ultrasonic, 30% ethanol, 50°C, 10 minutes |
| VIII | Ultrasonic, 40% ethanol, 50°C, 10 minutes |
Flexural Strength and Flexural Modulus Test
Following ISO 20795-2 [20], three measurements of width and height of each specimen were performed using a vernier caliper at three equally spaced points along the long axis, and stored in water at 37±1°C for 50±2 hours [20]. Each specimen was placed on the supports of a flexural jig, 50±0.1 mm apart, immersed in a water bath at 37°C and loaded in a universal testing machine (Shimadzu EZ-S, Shimadzu, Kyoto, Japan) at acrosshead speed at 5±1 mm per minute until fracture. The flexural strength and flexural modulus were calculated using the following formulae:
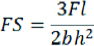
Where:
FS=flexural strength (MPa)
F=load at fracture (N)
l=distance between supports (mm)
b=mean of specimen width, measured immediately prior to water storage (mm)
h=mean of specimen height, measured immediately prior to water storage (mm)
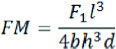
Where:
FM=flexural modulus (MPa)
F1=load (N) at a point in the straight line portion of the load/deflection curve
d=deflection (mm) at load F1
l, b, and h=as specified above
Vickers Microhardness Test
One fragment of each specimen broken from the flexural strength and flexural modulus tests was selected for microhardness testing. The Vickers microhardness (VHN) of each specimen was measured using a Vickers hardness tester (Future-tech FM-810, Future-tech, Kanagawa, Japan) under 25 gf load for 30 seconds [19]. The diagonal lengths were measured and automatically converted to VHN by the instrument. The measurements were performed five times on one fragment of each broken specimen and an average VHN value was calculated.
Water Sorption and Water Solubility Test
The water sorption and water solubility tests were performed per ISO 20795-2 [20]. Each disc-shaped specimen was placed in a rack inside a desiccator containing dried silica gel, stored in an oven at 37±1°C for 23±1 hours, transferred to a rack in a second desiccator and stored at 23±2°C for 60±10 minutes. Each specimen was weighed with an analytical balance to two decimal places. The specimens were replaced in the rack, returned to the first desiccator in the oven for another 23±1 hours, and reweighted. This cycle was repeated until the mass was constant (the mass loss was not more than 0.2 mg). The final mass of each specimen (m1) was recorded, and the volume, V, calculated using the mean of three diameter measurements and five thickness measurements. The diameters were measured at three equally spaced locations around the circumference and the thickness was measured at the centre and at four equally spaced locations of each specimen.
The specimens were immersed in water at 37±1°C for 7 days, removed from the water, wiped with a clean dry towel, waved in the air for 15±1 seconds, and weighed (m2) at 60±10 seconds after removal from the water bath. Each specimen was replaced in the rack inside the first desiccator and the cycle of m1 measurement was repeated until a constant mass was reached (m3). The water sorption (wsp) and water solubility (wsl) were calculated using the following formulae:
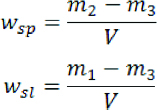
Statistical Analysis
Statistical analysis was performed using SPSS for Windows version 22.0 (IBM, New York, NY, USA) at a 95% confidence interval. The normality test was performed by Kolmogorov-Smirnov test. If the data were normally distributed, one-way ANOVA was used to find significant differences in properties, followed by Tukey’s HSD post hoc test. In the case of non-normal distribution, the Kruskal-Wallis test was used to identify significant differences, followed by the Mann-Whitney U test.
Results
Means and standard deviations of flexural strength and flexural modulus. Means with the same letter are not significantly different.
| Group | Treatment | Flexural strength (MPa) | Flexural modulus (MPa) |
|---|
| I | Control | 65.6±1.23a | 2,092.7±27.1A |
| Water, 25°C |
| II | 24 h | 67.8±1.10a,b | 2,206.9±34.5D,E |
| III | 72 h | 65.8±2.53a | 2,129.4±44.7A,B |
| Ultrasonic bath, 50°C |
| IV | Water | 69.1±1.62b | 2,182.7±34.5B,C,D,E |
| V | 10% ethanol | 67.0±2.63a,b | 2,138.7±41.6A,B,C |
| VI | 20% ethanol | 67.5±1.64a,b | 2,236.3±37.2E |
| VII | 30% ethanol | 66.9±1.48a,b | 2,158.3±68.9B,C,D |
| VIII | 40% ethanol | 68.5±1.87b | 2,197.8±50.4C,D,E |
The flexural strength of the Group I specimens demonstrated the lowest mean value, while the specimens from Group IV had the highest mean value. Those of Groups IV and VIII were significantly higher than those of Groups I and III; however, those of Groups II, V, VI, and VII were not.
The flexural modulus of the Group I specimens had the lowest mean value, while the specimens from Group VI demonstrated the highest mean value. Those of Groups II, IV, VI, VII, and VIII were significantly higher compared with Group I, while those of Group III and Group V were not significantly different.
Means and standard deviations of microhardness. Means with the same letter are not significantly different.
| Group | Treatment | Microhardness (VHN) |
|---|
| I | Control | 13.2±0.25a |
| Water, 25°C |
| II | 24 h | 13.8±0.63a |
| III | 72 h | 13.3±0.34a |
| Ultrasonic bath, 50°C |
| IV | Water | 13.6±0.41a |
| V | 10% ethanol | 13.3±0.46a |
| VI | 20% ethanol | 13.3±0.67a |
| VII | 30% ethanol | 13.2±0.46a |
| VIII | 40% ethanol | 13.6±0.43a |
The Vickers hardness number (VHN) of the Group VII specimens had the lowest mean value, while that of Group II was the highest mean value. However, the results did not show a significant difference in VHN of the experimental groups, compared with their controls.
Means and standard deviations of water sorption and water solubility. Means with the same letter are not significantly different.
| Group | Treatment | Water sorption (μg/mm3) | Water solubility (μg/mm3) |
|---|
| I | Control | 19.4+0.54a,b | 0.44+0.18A |
| Water, 25°C |
| II | 24 h | 19.2+0.26a,c | 0.48+0.19A |
| III | 72 h | 19.2+0.49a | 0.48+0.19A |
| Ultrasonic bath, 50°C |
| IV | Water | 19.8+0.17b | 0.89+0.25B |
| V | 10% ethanol | 19.6+0.28a,b | 0.53+0.16A |
| VI | 20% ethanol | 18.7+0.16c,d | 0.35+0.10A |
| VII | 30% ethanol | 18.3+0.40d | 0.43+0.09A |
| VIII | 40% ethanol | 18.6+0.32d | 0.48+0.12A |
The water sorption of the Group VII specimens demonstrated the lowest mean value, while the specimens from Group IV presented the highest mean value. Those of Groups VI, VII, and VIII showed significantly lower than Group I, while those of Groups II, III, IV and V were not significantly different.
For water solubility, the Group VI specimens showed the lowest mean value, while the specimens from Group IV had the highest mean value. However, there were no significant differences in water solubility between experimental groups compared with their controls, except that of Group IV that was significantly higher compared with Group I.
Discussion
In the present study, MMA-based orthodontic base-plate material immersed in an ultrasonic bath with both water and ethanol solution at 50°C demonstrated improved or maintained flexural properties, compared with the untreated groups. These results agree with those of Neves CB et al., that showed improved flexural strength of acrylic resin after immersion in 20% and 50% ethanol solutions and decreased flexural strength after immersion in a 70% ethanol solution [19]. Moreover, these results correspond with the investigation of Kobnithikulwong N et al., where the immersion of the prosthodontic auto-polymerized acrylic resin in an ultrasonic bath with ethanol solution did not affect flexural properties [22].
Many previous studies showed that post-polymerization treatments enhanced some mechanical properties of acrylic resins, such as flexural strength [12, 19], flexural modulus [23], and microhardness [1,12,19,24]. This improvement can be explained in two ways. First, these treatments promote further polymerization, which causes more cross-links between the polymer chains [15, 25]. Second, these treatments facilitate the release of the residual monomer that act as a plasticizer and lower the glass transition temperature [13].
The use of ultrasonic bath immersion in this study improved the flexural properties, but not microhardness. Charasseangpaisarn T et al., found that using ultrasonic treatment with water for 5 minutes can effectively reduce the level of residual monomer in the auto-polymerized acrylic resin used in prosthodontics, especially when performed at a low frequency [26]. The present study used an ultrasonic bath with various concentrations of ethanol in water as a post-polymerization treatment to investigate the effect on the physical properties of the resin. Ultrasonic treatment may affect the amount of residual monomer in acrylic resin in two ways; first, the propagation of ultrasound pressure and cavitation results in acceleration and internal diffusion of residual monomer; and second, exploding energy from cavitation may induce polymerization of the residual monomer [26].
Using ethanol solution for post-polymerization treatment also enhances the reduction of residual monomer in acrylic resins. Neves CB et al., found that the amount of residual monomer in acrylic reline resins was lower after immersion in higher ethanol concentrations [19]. This phenomenon can be explained by the Hildebrand solubility parameter (δ) [27], whereby two solutions of similar δ-value (MPa1/2) tend to be miscible. The δ-value PMMA monomer is approximately 16.0, while that of ethanol is 26.0. The latter value is closer to that of water (47.9). Furthermore, using a high temperature is an important factor in post-polymerization treatment, because of its capacity to stimulate additional polymerizations [15,25]. Therefore, using an ethanol solution at 50°C combined with ultrasonic immersion may effectively reduce the amount of the residual monomer in acrylic resins [22].
Urban VM et al., evaluated the effect of water bath post-polymerization on the mechanical properties of hard reline resins and found that flexural strength and microhardness increased [12]. Different types of acrylic resins and the techniques used in our and their studies may have contributed to these disparate results. We used an orthodontic acrylic resin mixed using a spray-on technique, while they used a prosthodontic acrylic resin mixed with a dough technique. A report found that mixing acrylic resin with the spray-on technique resulted in more porosity compared with the dough mixing technique [28]. These results imply that the former technique resulted in a higher volume of water migrating into the polymer network compared with the latter. The water in a polymeric acrylic network can reduce the mechanical properties by its plasticizing effect [29].
The present study found a decrease in water sorption with a higher ethanol concentration in solution. Dogan A et al., found a positive correlation between residual monomer and water sorption, because a higher amount of residual monomer led to increased porosities in the polymer mass [8]. When immersing the acrylic resin in water, residual monomer leaches out and replaced with water [30,31]. The resulting difference may be due to the effectiveness of an ultrasonic combined with ethanol solution. Additional polymerization of the residual monomer occurred, reducing the porosity in the polymer mass. Although the water sorption was decreased, the water solubility was unchanged. This may be a cause of post-treatment reduction of the water-soluble compound in acrylic resins.
To determine the most effective post-polymerization treatment, whatever best reduces the level of residual monomer should be primarily considered. However, the effect of post-polymerization treatment on physical properties should not be ignored. In this study, the physical properties of the groups met the MMA-based orthodontic base-plate material’s ISO standard. Thus, ultrasonic bath immersion with either warm water or a range of ethanol concentrations (10%-40%) can be effectively used to reduce the residual monomer in a short period of time.
Limitation
In this study, the specimens were fabricated manually. Therefore, the dimensions of each specimen might not be precise in some areas of the specimen. Mechanical fabrication is suggested to generate samples with precise dimensions.
Conclusion
Ultrasonic immersion in either water or ethanol solution at 50°C maintains or increases the flexural strength and modulus of an MMA-based orthodontic base-plate material, and do not significantly affect microhardness. Although the water sorption was significantly decreased with the ultrasonic treatment in 20-40% ethanol solutions, the water solubility was not affected by these treatment. Under our experimental conditions, ultrasonic treatment in water or ethanol solution had no adverse effects on the physical properties of an MMA-based orthodontic base-plate material. Immersion of the material in water or ethanol solutions at 50°C for 10 minutes can be considered an effective postpolymerization treatment contributing to decreased material toxicity. However, future study should focus on the cytotoxicity of MMA-based orthodontic base-plate materials after receiving ultrasonic treatment.