Mucoepidermoid Carcinoma of Lung Presenting as a Peripheral Cavitary Lesion
KO Rohit1, Jacob Baby2, K Praveen Valsalan3, Elizabeth Sunila4, Mitchelle Lolly5
1 Specialist, Department of Pulmonology, Aster Medcity, Kochi, Kerala, India.
2 Consultant, Department of Pulmonology, Aster Medcity, Kochi, Kerala, India.
3 Consultant, Department of Pulmonology, Aster Medcity, Kochi, Kerala, India.
4 Consultant, Department of Pulmonology, Aster Medcity, Kochi, Kerala, India.
5 Specialist, Department of Pulmonology, Aster Medcity, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. KO Rohit, Specialist, Pulmonology, Aster Medcity, Kochi-682034, Kerala, India.
E-mail: rohitkallukadavil@gmail.com
Mucoepidermoid Carcinoma (MEC) is a salivary gland neoplasm which originates from minor salivary glands in proximal bronchi. They are usually central in location adjacent to large airways. Here, we report a case of MEC which presented as a peripheral lesion with central cavitation. Patient underwent left upper lobectomy and histopathology was positive for p63, p40, CK5/6, CK7 and negative for TTF-1, Napsin A, with overall features in favour of high grade MEC. Post procedure he was stable and was later advised for chemotherapy.
Lung cancer, Mucoepidermoid tumour of lung, Salivary gland tumour
Case Report
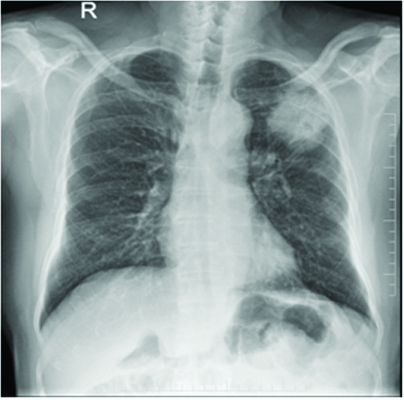
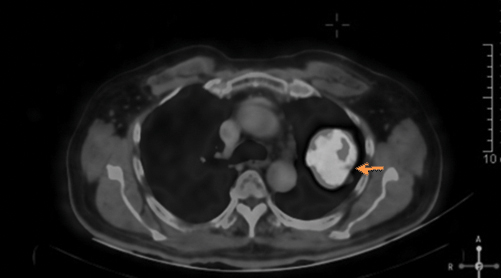
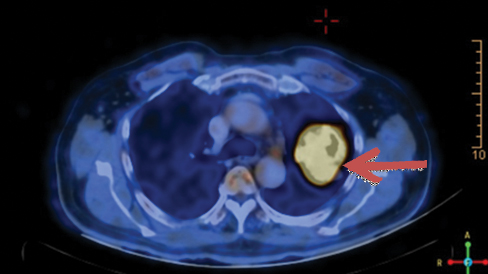
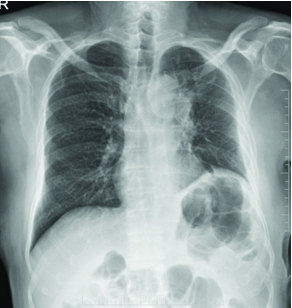
A 78-year-old male patient ex-smoker with 20 pack years, diabetic and hypertensive presented to the Department of Pulmonology with complaints of cough for two months duration. Cough was associated with mild mucoid sputum but he never reported blood stained sputum. He was evaluated in a local hospital where Computed Tomography (CT) scan showed left upper lobe lung lesion and was referred for further evaluation and management. On clinical examination he had clubbing but respiratory system examination did not reveal any abnormalities. Blood investigations were unremarkable. Chest X-ray showed left upper lobe lesion [Table/Fig-1]. CT scan revealed heterogeneous solid enhancing spiculated 58x40x44 mm mass lesion with central cavitation in left upper lobe apicoposterior segment [Table/Fig-2]. PET scan revealed FDG uptake in the lesion with no evidence of metastasis [Table/Fig-3]. Possible differential diagnosis made at this stage was cavitating lung carcinoma like adenocarcinoma, Infective aetiologies like tuberculosis or fungal infection. A CT guided biopsy of the lesion was done which revealed features in favour of malignancy but had low cellularity to characterise. Bronchoscopy did not reveal any intraluminal growth. Broncho Alveolar Lavage (BAL) was negative for tuberculosis, fungal growth and cytology did not show any atypical cells. Since the CT guided biopsy reported as suspicious malignancy it surgical removal of the lesion was decided. Left upper lobectomy with clearance of hilar and inferior pulmonary ligament nodes were done through Video assisted thoracoscopic surgery. Postoperative period was uneventful, chest X-ray showed post lobectomy status [Table/Fig-4] and he was discharged after few days.
Chest X-ray showing left upper lobe lesion.
CT thorax with lung window showing left upper lobe cavitatory lesion. (Orange coloured arrow)
PET CT showing FDG avid left upper lobe lesion (Brown coloured arrow).
Chest X-ray on postoperative day 7.
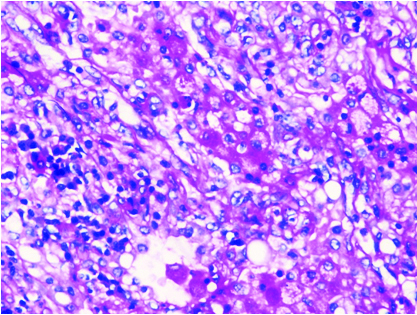
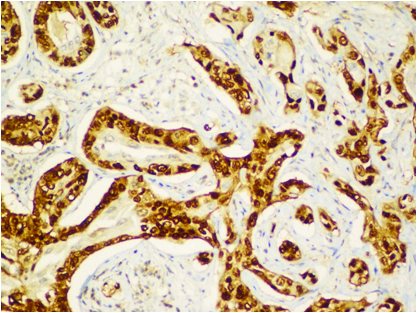
Cut section of lung specimen showed irregular grey white lesion 6x5x4 cm abutting the pleura, and Haematoxylin and Eosin stain showing lung tissue with a solid invasive malignant neoplasm arranged in adenomatous configuration and in small nests. The tumour cells were pleomorphic with marked nuclear atypia, peripherally clumped chromatin conspicuous nucleoli and moderate/vacuolated cytoplasm [Table/Fig-5,6]. Few cells showed intense eosinophilia and individual cell keratinisation and small nests of keratinised cells. Visceral pleural invasion was present. Frequent mitotic activity noticed (8-10/10HPF). Focal areas show lymphocytic infiltrate around tumour population. No evidence of lympho-vascular invasion. Alcian blue and D-PAS [Table/Fig-7] highlights the mucin in vacuolated cells. Tumour cells were positive for p63, p40, CK5/6, CK7 [Table/Fig-8,9,10 and 11] and negative for TTF-1, Napsin A. Negative stain for TTF-1 ruled out primary lung malignancy. Presence of Carcinoma specific p63 and CK 5/6 leads to the possibility of epithelial tumour. Vacuolated cells with Alcian blue and PAS showed presence of muciphages which are commonly seen in Salivary gland tumour. Overall features were suggestive of high grade MEC. In view of visceral invasion he was advised systemic chemotherapy with Pemetrexed and carboplatin. On follow-up after two months patient was symptomatically better.
Histopathology 100 X magnification showing H&E staining, tumour with lymphocytic infiltrates and muciphages (Black arrow on left bottom).
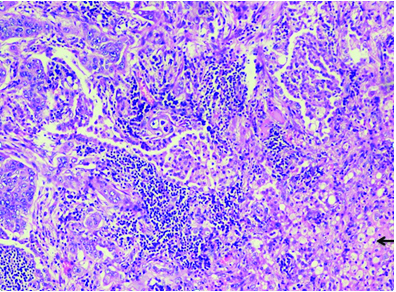
Histopthology-20x with H&E staining showing normal lung tissue (Blue arrow) with tumour (Black arrow).
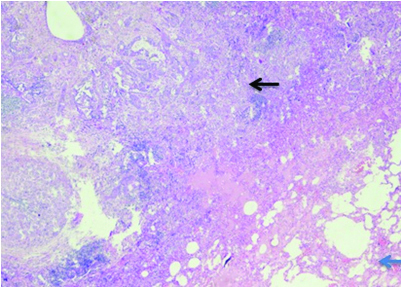
Special stain PAS (Periodic acid- Schiff) positivity.
Immunohistochemistry showing p63 positivity.
Immunohistochemistry showing p40 positivity.
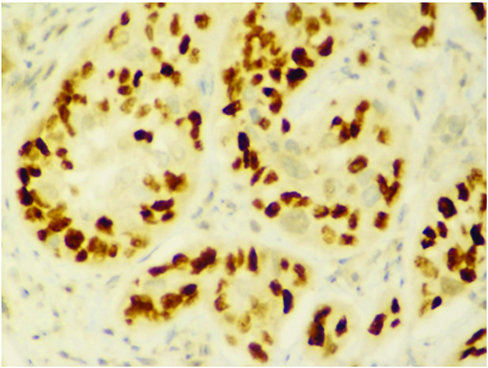
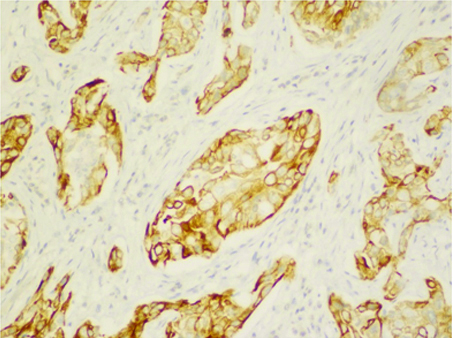
Immunohistochemistry showing CK5/6 positivity.
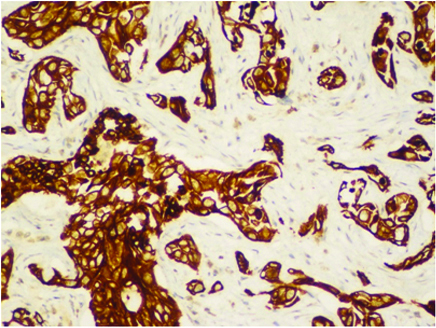
Immunohistochemistry showing CK7 positivity.
Discussion
MEC is a rare tumour in lung and was first described by Smetana and Liebow [1]. It is a tumour of bronchial gland origin with incidence of 0.1 to 0.2% of among lung cancers [2] and occurs in relatively younger age group [3,4]. It is a salivary gland type tumour and there is no definite association with smoke or occupational exposure [5].
MEC arises from central bronchial glands [6] and appear as solid lesions adjacent to larger airways. To our knowledge, this is the only one reported case of MEC presenting as a parenchymal lesion away from main bronchi and associated with cavitation. For cavitory lung mass in a patient with significant smoking history, the possible differentials are malignancy especially squamous cell or adenocarcinoma and tuberculosis [7]. Sputum and BAL was negative for Gene XPERT TB PCR. Histopathological and Immunohistochemistry findings were in favour of high grade MEC.
MEC may appear as polypoid, pink-tan in colour, soft with cystic changes and glistening appearance [5,6]. Tumour size at diagnosis may range from 0.5 to 6 cm. Patient can present with symptoms of cough, dyspnoea, haemoptysis, wheezing or fever. Histologically they are thought to arise from minor salivary glands lining the tracheobronchial tree [8]. MEC is classified as high or low grade depending on the histological appearance, mitotic activity, cellular atypia, necrosis and local invasion. Low grade tumours have cystic and solid areas comprising small glands, tubules and cysts of mucin secreting epithelial cells with infrequent mitotic activity. Non keratinising squamoid cells can also be seen in sheet like pattern. High grade MEC are relatively rare and have squamoid cell predominance with minor component of mucin secreting elements lining with nuclear atypia, high mitotic activity and increased nuclear cytoplasmic ratio [5,6]. In our case mitotic activity was 8-10/10HPF in favour of high grade tumour.
Well differentiated tumours have benign behaviour [9]. Low grade tumours have a better prognosis. However, surgery is the preferred treatment of pulmonary MEC. There is no proven effect of chemotherapy or radiotherapy for MEC [5,6]. In view of less number of cases there are no consensus regarding the treatment of high grade MEC [10]. In Shen C et al., study of eight MEC showed good response to surgery however in high grade cancer surgery resulted in significantly worse prognosis [11]. Xi J et al., study showed that age, grade of MEC, lymph node metastasis and TNM stage correlate with survival of patients [9]. Treatment with EGFR TKIs have shown benefit in some studies [12]. Studies have proven that pulmonary MEC may have t (11;19) translocation with fusion of oncogene (CRCT1-MAML2) [13-15]. Further studies are needed to assess the possibility of targeted chemotherapy in these patients.
Conclusion
Primary MEC of lung is a rare type of lung cancer. Even though it arises from central air ways, it can present as a peripherally located cavitating lung lesion. Biopsy with immunohistochemistry can confirm diagnosis. In low grade tumours, surgery is the treatment of choice. In view of rarity of cases and lack of data and role of chemotherapy is yet to be determined.
[1]. Smetana HF, Iverson L, Swan LL, Bronchogenic carcinoma; an analysis of 100 autopsy casesMil Surg 1952 111(5):335-51. [Google Scholar]
[2]. Pulmonary mucoepidermoid carcinoma in Chinese population: a clinicopathological and radiological analysis. - PubMed - NCBI [Internet]. [cited 2018 Jun 25]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26045810 [Google Scholar]
[3]. Yousem SA, Hochholzer L, Mucoepidermoid tumours of the lungCancer 1987 60(6):1346-52.10.1002/1097-0142(19870915)60:6<1346::AID-CNCR2820600631>3.0.CO;2-0 [Google Scholar] [CrossRef]
[4]. Kitada M, Matsuda Y, Sato K, Hayashi S, Ishibashi K, Miyokawa N, Mucoepidermoid carcinoma of the lung: a case reportJ Cardiothorac Surg 2011 6:13210.1186/1749-8090-6-13221985459 [Google Scholar] [CrossRef] [PubMed]
[5]. Travis WD, Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart [Internet][cited 2017 Oct 15]. Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/ [Google Scholar]
[6]. Belgod SR, Reddy RHV, Kumar SP, Mucoepidermoid carcinoma of the lung: a rare entityOxf Med Case Rep 2015 2015(2):203-05.10.1093/omcr/omv01225988080 [Google Scholar] [CrossRef] [PubMed]
[7]. Gadkowski LB, Stout JE, Cavitary Pulmonary DiseaseClin Microbiol Rev 2008 21(2):305-33.10.1128/CMR.00060-0718400799 [Google Scholar] [CrossRef] [PubMed]
[8]. Klacsmann PG, Olson JL, Eggleston JC, Mucoepidermoid carcinoma of the bronchus: an electron microscopic study of the low grade and the high grade variantsCancer 1979 43(5):1720-33.10.1002/1097-0142(197905)43:5<1720::AID-CNCR2820430523>3.0.CO;2-Q [Google Scholar] [CrossRef]
[9]. Xi J, Jiang W, Lu S, Zhang C, Fan H, Wang Q, Primary pulmonary mucoepidermoid carcinoma: an analysis of 21 casesWorld J Surg Oncol 2012 10:23210.1186/1477-7819-10-23223114230 [Google Scholar] [CrossRef] [PubMed]
[10]. Alsidawi S, Morris JC, Wikenheiser-Brokamp KA, Starnes SL, Karim NA, Mucoepidermoid carcinoma of the lung: a case report and literature reviewCase Rep Oncol Med [Internet] 2013 [cited 2017 Oct 20] 2013:625243Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834989/10.1155/2013/62524324303221 [Google Scholar] [CrossRef] [PubMed]
[11]. Shen C, Che G, Clinicopathological analysis of pulmonary mucoepidermoid carcinomaWorld J Surg Oncol 2014 12(1):3310.1186/1477-7819-12-3324507476 [Google Scholar] [CrossRef] [PubMed]
[12]. Han S-W, Kim H-P, Jeon YK, Oh D-Y, Lee S-H, Kim D-W, Mucoepidermoid carcinoma of lung: Potential target of EGFR-directed treatmentLung Cancer 2008 61(1):30-34.10.1016/j.lungcan.2007.11.01418192072 [Google Scholar] [CrossRef] [PubMed]
[13]. Liu X, Adams AL, Mucoepidermoid carcinoma of the bronchus: a reviewArch Pathol Lab Med 2007 131(9):1400-04. [Google Scholar]
[14]. O’Neill ID, t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinomaOral Oncol 2009 45(1):2-9.10.1016/j.oraloncology.2008.03.01218486532 [Google Scholar] [CrossRef] [PubMed]
[15]. Achcar RDOD, Nikiforova MN, Dacic S, Nicholson AG, Yousem SA, Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinomaHum Pathol 2009 40(6):854-60.10.1016/j.humpath.2008.11.00719269006 [Google Scholar] [CrossRef] [PubMed]