Prosthetic Rehabilitation of a Large Frontal Cranial Defect-A Case Report
Mitalee Ajit Mopkar1, Meena Ajay Aras2, Vidya Chitre3, Ashwin R Mysore4, Kennedy Mascarenhas5
1 Postgraduate Student, Department of Prosthodontics, Goa Dental College and Hospital, Goa, India.
2 Professor and Head, Department of Prosthodontics, Goa Dental College and Hospital, Goa, India.
3 Professor, Department of Prosthodontics, Goa Dental College and Hospital, Goa, India.
4 Lecturer, Department of Prosthodontics, Goa Dental College and Hospital, Goa, India.
5 Lecturer, Department of Prosthodontics, Goa Dental College and Hospital, Goa, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mitalee Ajit Mopkar, House No. 9/463 G “PREET”. Housing Board, Ganeshpuri, Mapusa, North Goa-403507, Goa, India.
E-mail: mitalee.mopkar@gmail.com
With an increased incidence of automobile accidents, head injuries in road traffic accident victims are common. In order to salvage the vital intra-cranial structures from the effects of trauma, emergency attention is necessary. Retrieval of crushed bone fragments or drainage of haematomas to relieve intracranial pressure leaves the patient with continuity defects and unprotected brain tissue. Due to the complex field of expertise involved, rehabilitation of such defects is challenging and requires interdisciplinary attention. This article reports a case of a large frontal cranial defect rehabilitated using a heat polymerised polymethyl methacrylate acrylic resin prosthesis.
Acrylic bone flap, Cranial prosthesis, Frontal defect rehabilitation, Maxillofacial prosthesis, Polymethyl methacrylate prosthesis
Case Report
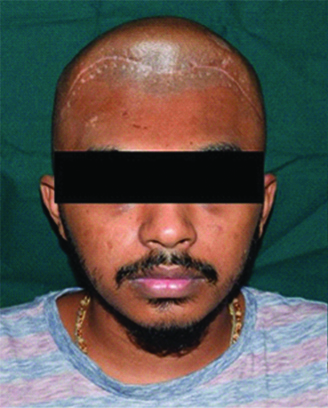
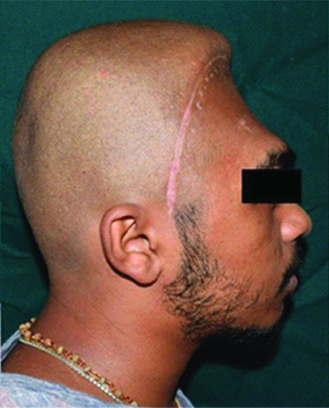
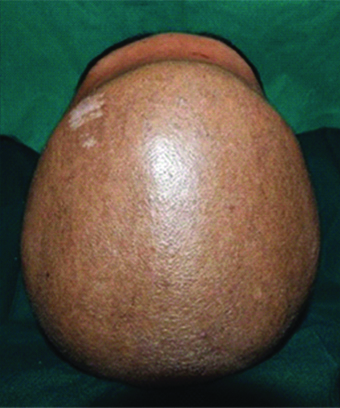
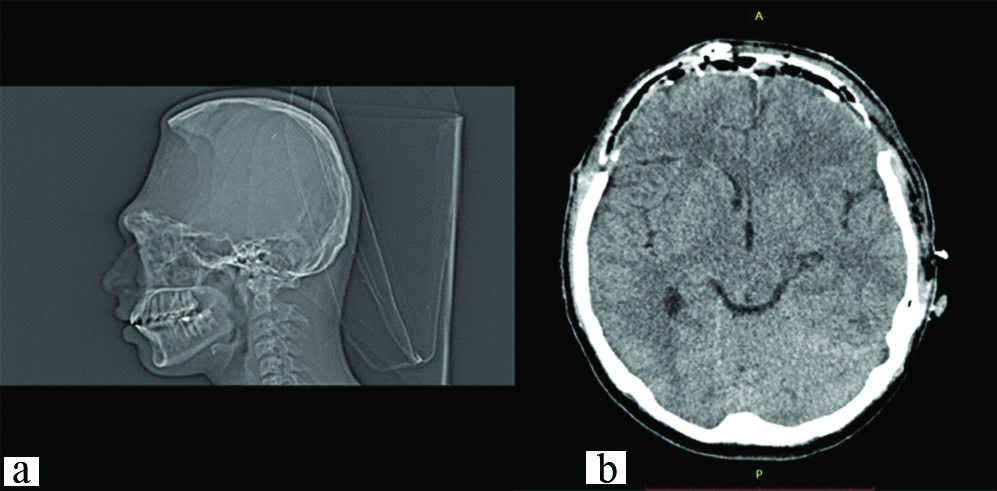
A 19-year-old male patient reported to the Department of Prosthodontics, with the chief complaint of an unpleasant appearance due to a defect in the forehead area of his face [Table/Fig-1,2 and 3]. The patient’s history revealed his involvement in a motor vehicle accident three months ago. The history also revealed removal of the frontal bone due to internal bleeding and hydrocephalus as a result of the trauma. During this period, the patient was advised to use a thick head cap for protection. Clinical and preoperative radiographic examination (anteroposterior view, CT scan) was carried out. The CT scan revealed a large radiolucent defect involving the entire frontal bone extending laterally to involve the anterior portion of the parietal bones on both the left and right side. Supero-inferiorly the defect extended from the frontal fontanelle to nasion [Table/Fig-4a]. The axial section taken at the level of the glabella showed a discontinuous cranial vault in the anterior region. The thin radiodense rim is suggestive of the anterior wall of the frontal sinus [Table/Fig-4b]. The retrieved bone flap was intact and therefore, preserved in a chlorhexidine gluconate and cetrimide antiseptic solution (Savlon, India) to be replicated. The flap was retrieved from the Department of Neurosurgery, Goa Medical College and Hospital and was then transported to the Department of Prosthodontics Goa Dental College and Hospital where it was stored until replication. Considering the size and location of the defect and due to financial constraints, a decision was made to fabricate the cranial prosthesis out of Polymethyl Methacrylate (PMMA) acrylic resin.
Preoperative frontal view.
Preoperative lateral view.
Preoperative birds eye view.
Preoperative CT Scan: a) sagittal section; b) axial section.
The bone flap was carefully cleaned with a disinfectant (2% glutaraldehyde) and coated with a thin, even layer of modelling wax (Deepti Dental products of India Pvt., Ltd.,) to smoothen out irregularities. A stainless steel flask was customised to facilitate laboratory processing. The waxed up flap was invested in the flask using Type III dental stone (Goldstone, Asian Chemicals, and India). Upon dewaxing, separating medium (Heat Cure Cold Mould Seal, Dental products of India), was painted onto the mould and the mould was packed with heat polymerising clear PMMA resin (Pro Base Hot, Ivoclar Vivadent). The assembly was bench cured and processed using the long curing cycle of eight hours at 74°C [Table/Fig-5a-d]. The prosthesis was divested and immersed in distilled water overnight. The prosthesis was then finished and polished. Perforations of 2.5mm diameter were made all over the body of the prosthesis, 3mm apart from each other, leaving a 5mm margin for fixation. In order to facilitate radiographic detection, shallow rectangular troughs were drilled along the margin measuring 4×0.5×1mm into which light cure composite resin (Tetric N Ceram, IvoclarVivadent) was packed [Table/Fig-5d]. The contour was verified externally by a quick visual examination and the prosthesis was immediately delivered to the neurosurgeons for further surgical procedures.
a) Waxed up prosthesis; b) Invested prosthesis; c) Dewaxed mould; d) Acrylised and finished prosthesis.

During surgery, the defect was exposed through the existing scar and the autoclaved prosthesis was fixed to the adjacent bone margin. The patient was followed-up over a period of six months post-surgery. No complications were observed and the patient was satisfied with the outcome of the procedure [Table/Fig-6].
Postoperative view: a) frontal; b) lateral; c) bird’s eye.

Discussion
Defects involving the cranial vault could be a result of congenital deformities, trauma, decompressive craniectomies, and/or loss of bone flap due to infection [1-3]. Apart from the patient’s physical appearance, a large surface of the brain remains unprotected due to absence of bone which is a greater concern. Patients with large cranial defects often present with neurological symptoms headaches, dizziness, irritability, epilepsy, discomfort, and psychiatric symptoms which are known to improve with rehabilitation [4]. The repair of these defects is known as cranioplasty and involves multidisciplinary planning by craniofacial surgeons, neurosurgeons as well as prosthetic clinicians. One of the greatest benefits of cranioplasty is the improvement in neurologic function, which is attributed to changes in brain physiology resulting from improved cerebral blood flow, cerebrovascular reserve capacity and cerebral glucose metabolism [4]. A variety of materials can be used for fabrication of cranial prostheses. Cranial defect reconstruction can be carried out using autologous and alloplastic bone grafts, resin materials like PMMA and Polyhydroxyethyl methacrylate, Titanium in the form of plates and reinforcement meshes, hydroxyapatite, silicones, Polyethyl Ether Ketone (PEEK) etc., [5,6]. According to Cho YR et al., the ideal biomaterial should be biocompatible, radiolucent, easily moulded, strong enough to endure trauma, able to maintain volume, stable over time and osteoactive [7]. The most ideal material in terms of biocompatibility and osteoactivity to replace lost tissue would be an autologous bone graft. Although autologous bone grafts have the ability of complete biological integration, their use is limited and are not suitable in large defects due to donor site morbidity and the risk of resorption after implantation [7]. The bone flaps obtained from a bone bank bear an inherent risk of infection [5,8].
Titanium is highly biocompatible as it has a low rate of corrosion, low rate of toxicity and inflammatory reactions [3,5]. However, it is expensive, relatively difficult to fabricate and adjust intraoperatively [6,8]. Being metallic, they also bear the disadvantage of high heat conduction leading to discomfort especially in warmer climatic conditions [6].
Despite multiple options being available, heat polymerised PMMA acrylic is used widely and has many advantages [6,9]. It is readily available in most hospital setups and is inexpensive. It can be easily moulded into a desired shape and the hardened implant is strong, light and inert to adjacent tissues. The finished prosthesis is easy to adjust, and secure to the adjacent skull bone with screws and bioplates [9]. With all these benefits taken into consideration the present case was rehabilitated using a customised prefabricated cranial prosthesis from heat polymerised PMMA acrylic as opposed to cranioplasties carried out in-situ using self-cure PMMA acrylic. Therefore, this technique had the advantage of shorter intraoperative time, complete polymerisation and no irritation from exothermic heat of setting of PMMA, eliminating the potential threat of thermonecrosis [8]. The presence of residual monomer which could cause cytotoxicity is greatly decreased by processing PMMA through a long curing cycle of eight hours at 74°C and is decreased even further by immersion of the prosthesis in distilled water thus minimising its content even further [10-13].
Since the prosthesis was an exact replica of the patient’s own bone flap, the contours were accurately replicated. The impression and trial appointments were not necessary which shortened the number of patient visits. The perforations incorporated into the prosthesis allows the fluid accumulated to seep out into the sub-galeal space, permits and encourage adhesions between the prosthesis and the soft tissue, and maintains adequate blood supply to the overlying scalp [11,14]. It also reduces the risk of development of an epidural haematoma [3].
The major disadvantage with PMMA is the rate of infection which is approximately 5%, especially in patients who have had a history of simultaneous cranial and orbital reconstructions and a history of previous infections [6,15]. PMMA being an inert and fixed substance does not adapt to the changing craniofacial skeleton making it a poor treatment option in growing children [15]. Methyl methacrylate being non-porous, bone ingrowth cannot be expected in cases such as these.
The limitation of the present case report was that no hypersensitivity testing was done.
Conclusion
In the case reported, the rehabilitation of the patient using a conventional material and technique gave excellent esthetic results. Thus, PMMA can be considered as a material that can successfully be used as a treatment option if conditions limit the use of other alternatives like autogenous grafts or titanium. Meticulous treatment planning involves weighing the pros and cons of all the material options available and appropriate clinical correlation to achieve the best results.
[1]. Beumer III J, Firtell DN, Curtis TA, Current concepts in cranioplastyJ Prosthet Dent 19791 42(1):67-77.10.1016/0022-3913(79)90332-9 [Google Scholar] [CrossRef]
[2]. Sane VD, Kadam P, Jadhav A, Saddiwal R, Merchant Y, Multidisciplinary approach for reconstruction of cranial defect with polymethyl methacrylate resin reinforced with titanium meshJ Indian Prosthodont Soc 2016 16:294-97. [Google Scholar]
[3]. Sharma A, Tiwari B, Ladha K, Naik D, Prosthetic rehabilitation of a traumatic fronto-parietal skull bone defect with titanium cranial prosthesisIndian Dental Journal 2015 7:7-21. [Google Scholar]
[4]. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT, Cranioplasty: Cosmetic or therapeutic?Surg Neurol 1997 47:238-41.10.1016/S0090-3019(96)00013-4 [Google Scholar] [CrossRef]
[5]. Neumann A, Kevenhoerster K, Biomaterials for craniofacial reconstructionGMS Curr Top Otorhinolaryngol Head Neck Surg 2009 8:08 [Google Scholar]
[6]. Spetzger U, Vougioukas V, Schipper J, Materials and techniques for osseous skull reconstructionMinimally Invasive Therapy & Allied Technologies 2010 19(2):110-21.10.3109/1364570100364408720166839 [Google Scholar] [CrossRef] [PubMed]
[7]. Cho YR, Gosain AK, Biomaterials in craniofacial reconstructionClinics in Plastic Surgery 2004 31(3):377-85.10.1016/j.cps.2004.03.00115219744 [Google Scholar] [CrossRef] [PubMed]
[8]. Abdulai A, Iddrissu M, Dakurah T, Cranioplasty using polymethyl methacrylate implant constructed from an alginate impression and wax elimination techniqueGhana Med J 2006 40(1):18-21. [Google Scholar]
[9]. Werndle MC, Crocker M, Zoumprouli A, Papadopoulos MC, Modified acrylic cranioplasty for large cranial defectsClinical Neurology and Neurosurgery 2012 114(7):962-64.10.1016/j.clineuro.2012.02.01922402199 [Google Scholar] [CrossRef] [PubMed]
[10]. Tsuchiya H, Hoshino Y, Tajima K, Takagi N, Beaching and cytotoxicity of formaldehyde and methylmethacrylate from acrylic resin denture base materialsJ Prosthet Dent 1984 71:618-24.10.1016/0022-3913(94)90448-0 [Google Scholar] [CrossRef]
[11]. Simon P, Mohan J, Selvaraj S, Saravanan BS, Pari P, Craniofacial Prosthetic Reconstruction Using Polymethyl Methacrylate Implant: A Case ReportThe Journal of Indian Prosthodontic Society 2014 14(1):303-07.10.1007/s13191-014-0361-326199536 [Google Scholar] [CrossRef] [PubMed]
[12]. Anusavice JK, Phillip’s Science of Dental Materials (Ch-22) 2003 11th ednSt. LouisElsevier:733 [Google Scholar]
[13]. Austin AT, The level of residual monomer in acrylic denture base materialsBritish Dent J 1980 18:281-86.10.1038/sj.bdj.48045116934785 [Google Scholar] [CrossRef] [PubMed]
[14]. Prithviraj DR, Anish G, Sumit K, Pujari M, Garg P, Combined surgical and prosthetic approach for rehabilitation of frontonasal defect using custom made titanium implant: a case reportJ Clin Exp Dent 2011 3(Suppl1):e382-85.10.4317/jced.3.e382 [Google Scholar] [CrossRef]
[15]. Gosain AK, Plastic Surgery Eductional Foundation DATA CommitteeBiomaterials for reconstruction of the cranial vaultPlastic and reconstructive surgery 2005 116(2):663-66.10.1097/01.prs.0000176289.05374.5b16079708 [Google Scholar] [CrossRef] [PubMed]