Diabetic foot is defined by World Health Organisation as “The foot of a DM patient that has the potential risk of pathologic consequences, including infection, ulceration, and/or destruction of deep tissues associated with neurologic abnormalities, various degrees of peripheral vascular diseases and/or metabolic complications of diabetes in lower limb” [1]. India is slowly progressing to the top of the world with the largest number of DM subjects and is being anticipated to be the “diabetes capital of the world”. According to the Diabetes Atlas 2013 published by the International Diabetes Federation, the number of people with DM in India was 65.1 million, which is expected to rise to 142.7 million by 2035 [2].
DM is responsible for approximately 80% of all non-traumatic amputations performed every year. After a major amputation, 50% of people will need to have the other limb amputated within two year’s time. People with a history of diabetic foot ulcer have a 40% greater 10-year death rate than people with DM alone [3]. This can be attributed to several social and cultural practices such as barefoot walking, inadequate facilities for care, education about diabetes, and poor socio-economic conditions.
Although, recent population-based data for Diabetic Foot Ulcer (DFU) is not available, it is estimated that approximately 45,000 legs are amputated every year in India [4-6]. DFU is difficult to treat, frequently get infected, and become a leading cause of diabetes-related hospital admission [7]. DM costs a whopping USD 548 billion in health expenditure globally in 2013. In a report published by Gupta S, an average Indian would spend approximately 1960 USD for the complete treatment of neuron-ischaemic foot [5].
Considering the substantial morbidity and mortality associated with LEA in people with DM [8], the ability to identify which patients hospitalised for a diabetic foot is at highest risk for this complication, could help clinicians to direct patients for special preventive efforts. This information also could help identify the baseline risk for LEA among patients admitted to a medical centre, allowing fairer comparisons of amputation rates.
Although the factors associated with DM people developing a foot ulcer are well defined, risk factors for amputation are less clear. Previous studies have identified independent risk factors that include a history of a foot ulcer, limb ischaemia, underlying bone involvement, presence of gangrene, deep wounds, older age, elevated inflammatory markers, poor glycaemic control, duration of DM, specific geographical region or ethnicity, nephropathy, and retinopathy [9-16]. The present study aims to develop a scoring system to predict the risk of LEA in patients with DFU.
Materials and Methods
One hundred fifty patients above the age of 18 years and both sexes with infected DFU attending outpatient department or admitted between October 2015 and November 2016 and ready to participate were included for this prospective, observational study after explaining potential advantages, and risks. Written informed consent was obtained from all the patients. Permission was obtained from the Ethical Committee (RECH/EC/2015-16/0705 dated 29/9/2015) and a scientific advisory committee of the institution. Exclusion criteria were foot ulcers due to peripheral vascular disease not associated with DM, all traumatic amputations, subjects with psychiatric illness, all DM patients with previously documented venous incompetence, subjects who were lost to follow-up, subjects who expired after taking part in the study within a stipulated period. Based on a previous study [5], setting an alpha error at 0.05, and power at 80%, the sample size of 150 patients was calculated by a formula [17]. A total of 203 patients presented with infected diabetic foot ulcer to the hospital. Out of which 29 refused to participate, three were psychiatric patients who were unable to give consent, seven patients expired within follow-up period and 14 patients lost to follow-up.
A pre-tested study proforma was used to collect data on demographics, duration of DM and treatment, self-care behaviours, neuropathic symptoms, the presence of intermittent claudication or pain at rest, past history of foot or leg ulcer and amputation. Physical examination with emphasis on the lower limbs was performed to assess for foot deformity (high arch or dropped foot), hammer/claw toe, equinus deformity, cavus deformity charcot deformity, hallux limitus, pedal oedema, callus, scars of previously healed ulcers, and amputation defects. Subjects were placed supine for at least five minutes. The systolic blood pressure of the brachial artery of both arms and the posterior tibial artery of both ankles were measured using a blood pressure cuff and Parks model 841-A pocket doppler probe. The highest arm pressure was used to calculate the Ankle Brachial Index (ABI) [18].
Examination of Ulcer
The ulcer was inspected for size, site, depth, presence of surrounding oedema, cellulitis, discharge, change in the colour of the surrounding skin, and surrounding temperature. Patients were asked about numbness, and a history was obtained on the number and sites of foot ulcers.
Sensory Neuropathy
This was tested using a 5.07/10 g Semmes Weinstein monofilament [19,20]. Testing sites included the dorsal surface between the base of the first and second toes, the plantar aspect of the first, third, fifth toes, the first, third, and fifth metatarsal heads, the medial and later midfoot, and the heel [21]. Each site was tested for one second and the patient was asked to respond as “touch” if he/she felt the monofilament touching the foot. If the subject correctly identified the sensation of monofilament at the testing site then the response was considered to be positive and testing proceeded to the next site. When subjects were unable to detect the applied pressure, they were tested again at the same level. The perception of the sense of touch at the tested site was registered to be negative if the patient failed to perceive the sensation even after three consecutive testing at the same site.
Motor Neuropathy
All the subjects were tested for motor neuropathy by assessing deep tendon reflexes and muscle strength [22]. Deep tendon reflexes like ankle reflex (achilles) and knee reflex (patella) were assessed. Muscle strength was assessed as the ability of the muscle to produce active movement against the examiner’s resistance and it was assessed by asking the patient to perform dorsiflexion of foot, plantar flexion of foot, leg flexion at knee and leg extension at knee for testing deep peroneal, tibial, sciatic, and femoral nerves respectively. The patient was considered to have motor neuropathy only if the neuropathy was symmetrical, bilateral and abnormality was proportionate to sensory neuropathy or else alternate cause for neuropathy was suspected.
Scoring Criteria
Sensory neuropathy: The response was graded into three grades. If the subject has no sensory neuropathy then it was graded as Grade 0. If subject failed to register perception of touch at one or two sites then it was graded as Grade 1. If the subject failed to register perception of touch at ≥3 sites then it was graded as Grade 2. Grades 0, 1, and 2 of sensory neuropathy were labelled as score 0, 1, and 2 respectively.
Motor neuropathy: If motor neuropathy was present the score was given as 2 whereas if it was absent the score was 0.
Grade of ulcer (Rutherford grades): Based on the ulcerations and gangrenous changes in lower limbs they were classified into Rutherford grades [23] asymptomatic (Grade 0), claudication present (Grade I), rest pain present (Grade II), minor tissue loss such as non healing ulcer, focal gangrene with diffuse pedal ischaemia (Grade III), and major tissue loss such as gangrene extended above TM level, functional foot no longer salvageable (Grade IV) and were labeled as scores 0, 1, 2, 3 and 4 respectively.
Duration of DM: Subjects were scored based on the duration of DM. Less than 10 years, 10 ≤20 years and >20 years were given scores as 1, 2, and 3 respectively.
Age: Subjects were scored based on age. Less than 50 years, 50≤65 years and >65 years were given scores as 1, 2, and 3 respectively.
HbA1c: Subjects were classified into four groups based on serum glycosylated haemoglobin (HbA1c) levels. <7% (53.0 mmol/mol), 7% (53.0 mmol/mol) <9.5% (80.5 mmol/mol), 9.5% (80.5 mmol/mol) <12.5% (113.5 mmol/mL) and ≥12.5% (113.5 mmol/mL) were given scores as 0, 1, 2 and 3 respectively.
Foot deformity: For the presence and absence of foot deformity subjects were allotted scores two and zero respectively.
History of previous amputations: Based on the history of previous amputations subjects were allotted scores. For the presence and absence of amputation, subjects were allotted scores 2 and 0 respectively.
ABI: The ratio of ankle to arm systolic blood pressure was calculated for each leg, and the lowest ratio was recorded as the ABI. Subjects were placed in two groups [18]; those with ABI between 0.9-1.3, and all others with ABI below 0.9 and above 1.3 and were allotted scores 0 and 2 respectively.
Depth of ulcer: Subjects were classified into four groups based on the depth of ulcer and the involvement of bone. Presence of osteomyelitis was determined by probe to bone test and X-ray images of involved foot. Scores 1, 2, 3 and 4 were allotted to persons having ulcer involving only subcutaneous tissue (Grade I), exposed tendons/vessels/muscles/nerve (Grade II), exposed bone (Grade III) and bony involvement/osteomyelitis (Grade IV) respectively.
Infectious Diseases Society of America (IDSA) infective score: IDSA infective score [24] defines the infective status of the diabetic foot ulcer. Based on the signs of local wound infection and the systemic response towards the subjects with DFUs were divided into four grades called IDSA severity levels. Scores 0, 1, 2 and 3 were allotted to persons having Grade I (uninfected), Grade II (mildly infected), Grade III (moderately infected) and Grade IV (severely infected) respectively.
Outdoor bare foot walking: Based on the history of outdoor bare foot walking subjects were scored 0 and 2 for the absence of barefoot walking and presence of barefoot walking respectively.
Comorbidities: Comorbidities considered in the study were diabetic nephropathy, congestive cardiac failure, diabetic retinopathy, HIV positive status, peripheral vascular diseases, liver diseases, ischaemic heart disease, history of stroke, hypertension, chronic pulmonary obstructive disease, and bronchial asthma. Scores 0 and 1 were allotted for absence and presence of comorbidity respectively.
The cumulative score of each subject calculated after adding the scores of their corresponding risk factors was called the total score for each subject. Subjects were adequately managed according to the guidelines of IDSA for diabetic foot.
Analysing the score of the subjects who required amputation gave us the range of the score that could predict amputations. During the study period, subjects were asked to follow-up after seven days, one month, and 12 months but the score was not recalculated at those visits. During these follow-up visits, subjects were examined for recurrence of ulcers, need for re-debridement and/or amputation. Subject who underwent re-debridement or amputations during follow-up period were included in the study, but the risk score at the time of second surgery was not calculated.
Statistical Analysis
The data on categorical variables are presented as n (% of cases). The statistical significance of the difference of categorical variables across two study groups (amputation required Vs amputation not required) was tested using the chi-square test or Fisher’s-Exact test. The optimal discriminating threshold cut-off of the total risk score for predicting the incidence of amputation was determined using Receiver Operating Characteristics (ROC) curve analysis. The measures of diagnostic efficacy indices such as sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and accuracy were calculated along with 95% CI for accuracy measures. The p-values less than 0.05 were considered to be statistically significant. All the hypotheses were formulated using two-tailed alternatives against each null hypothesis. The entire data were statistically analysed using Statistical package for social sciences (SPSS version 20.0, Inc. Chicago) for MS Windows.
Results
One hundred fifty patients were included in the study to develop a scoring system to predict the risk of LEA. In all 44/150 (29.3%) patients required amputation. It can be seen from [Table/Fig-1] that out of 44 patients who required amputation, 36/44 (81.8%) patients had total score ≥16. As the score increased the risk of amputation increased. All eight patients whose score was >25 underwent amputation. As depicted in [Table/Fig-2], percentage of patients who required amputation was significantly high in >65 years of age, duration of DM >20 years, sensory neuropathy Grade 2, presence of motor neuropathy, presence of deformity, IDSA infection Grade IV, past H/O amputation, ulcer depth Grade IV, HbA1c ≥12.5% (113.5 mmol/mol), ankle-brachial index ≤0.9 or ≥1.30, and Rutherford Grading IV, whereas there was no statistically significant difference in relation to H/O co-morbidity and H/O barefoot walking. It can be seen from [Table/Fig-3] that sensitivity was more than 90% for ulcer depth grade, sensory neuropathy and IDSA infection grade whereas specificity was more than 80% for past H/O amputation and HbA1c levels. As depicted in [Table/Fig-4], the cut-off (>16.5) by ROC highlighted in grey having relatively high sensitivity and specificity in predicting the incidence of amputation.
Distribution of amputations and total risk score (n=150).
| Total risk score | No. of cases | No. of amputations | % of amputations required |
|---|
| <10.0 | 38 | 0 | 0 |
| 10.0-15.0 | 64 | 8 | 12.5 |
| 16.0-19 | 24 | 13 | 54.1 |
| 20-24 | 16 | 15 | 93.7 |
| ≥25 | 8 | 8 | 100 |
| Total | 150 | 44 | 29.3 |
Risk factors and amputation.
| Variable | Amputation | Total n=150 (%) | p-value |
|---|
| Required n=44 (%) | Not required n=106 (%) |
|---|
| Age in years (%) |
| <50 | 5 (15.2) | 28 (84.8) | 33 (100.0) | 0.005 |
| 50≤65 | 26 (28.0) | 67 (72.0) | 93 (100.0) |
| >65 | 13 (54.2) | 11 (45.8) | 24 (100.0) |
| Duration of DM in years (%) |
| <10 | 13 (14.6) | 76 (85.4) | 89 (100.0) | 0.001 |
| 10≤20 | 21 (44.7) | 26 (55.3) | 47 (100.0) |
| >20 | 10 (71.4) | 4 (28.6) | 14 (100.0) |
| Grades of sensory neuropathy (%) |
| 0 | 1 (2.9) | 33 (97.1) | 34 (100.0) | 0.001 |
| 1 | 11 (15.9) | 58 (84.1) | 69 (100.0) |
| 2 | 32 (68.1) | 15 (31.9) | 47 (100.0) |
| Motor neuropathy (%) |
| Absent | 14 (17.3) | 67 (82.7) | 81 (100.0) | 0.001 |
| Present | 30 (43.5) | 39 (56.5) | 69 (100.0) |
| Deformity (%) |
| Absent | 19 (21.6) | 69 (78.4) | 88 (100.0) | 0.013 |
| Present | 25 (40.3) | 37 (59.7) | 62 (100.0) |
| IDSA infection grade (%) |
| I | 2 (16.7) | 10 (83.3) | 12 (100.0) | 0.009 |
| II | 9 (15.8) | 48 (84.2) | 57 (100.0) |
| III | 16 (37.2) | 27 (62.8) | 43 (100.0) |
| IV | 17 (44.7) | 21 (55.3) | 38 (100.0) |
| H/O amputation (%) |
| Absent | 23 (18.7) | 100 (81.3) | 123 (100.0) | 0.001 |
| Present | 21 (77.8) | 6 (22.2) | 27 (100.0) |
| Co-mobidity (%) |
| Absent | 17 (32.7) | 35 (67.3) | 52 (100.0) | 0.510 |
| Present | 27 (27.6) | 71 (72.4) | 98 (100.0) |
| Ulcer depth grade (%) |
| I | 1 (6.3) | 15 (93.7) | 16 (100.0) | 0.001 |
| II | 8 (8.7) | 84 (91.3) | 92 (100.0) |
| III | 16 (69.6) | 7 (30.4) | 23 (100.0) |
| IV | 19 (100.0) | 0 (0.0) | 19 (100.0) |
| H/O barefoot walking (%) |
| Absent | 21 (28.0) | 54 (72.0) | 75 (100.0) | 0.720 |
| Present | 23 (30.7) | 52 (69.3) | 75 (100.0) |
| HbA1c (%) |
| <7 (53.0 mmol/mol) | 8 (12.1) | 58 (87.9) | 66 (100.0) | 0.001 |
| 7.0 (53.0 mmol/mol)<9.5 (80.5 mmol/mol) | 3 (12.5) | 21 (87.5) | 24 (100.0) |
| 9.5 (80.5 mmol/mol) <12.5 (113.5 mmol/mol) | 17 (54.8) | 14 (45.2) | 31 (100.0) |
| ≥12.50 (113.5 mmol/mol) | 16 (55.2) | 13 (44.8) | 29 (100.0) |
| Ankle-brachial index (%) |
| 0.91-1.29 | 22 (21.0) | 83 (79.0) | 105 (100.0) | 0.001 |
| ≤0.9 or ≥1.30 | 22 (48.9) | 23 (51.1) | 45 (100.0) |
| Rutherford grading (%) |
| 0 | 7 (14.9) | 40 (85.1) | 47 (100.0) | 0.001 |
| I | 4 (16.0) | 21 (84.0) | 25 (100.0) |
| II | 4 (16.0) | 21 (84.0) | 25 (100.0) |
| III | 19 (44.2) | 24 (55.8) | 43 (100.0) |
| IV | 10 (100.0) | 0 (0.0) | 10 (100.0) |
Diagnostic efficacy of various risk factors.
| Diagnostic efficacy in percentage |
|---|
| Score | Risk factor | Sensitivity | Specificity | PPV | NPV | Accuracy [95% CI] |
|---|
| Score 1 | Age group | 88.6 | 26.4 | 33.3 | 84.8 | 44.7 (36.7-52.6) |
| Score 2 | Duration of DM | 70.5 | 71.7 | 50.8 | 85.4 | 71.3 (64.1-78.6) |
| Score 3 | Sensory neuropathy | 97.7 | 31.1 | 37.1 | 97.1 | 50.7 (42.7-58.7) |
| Score 4 | Motor neuropathy | 68.2 | 63.2 | 43.5 | 82.7 | 64.7 (57.0-72.3) |
| Score 5 | HbA1c | 36.7 | 87.7 | 55.2 | 76.9 | 72.7 (65.5-79.8) |
| Score 6 | Rutherford grading | 84.1 | 37.7 | 35.9 | 85.1 | 51.3 (43.3-59.3) |
| Score 7 | Deformity | 56.8 | 65.1 | 40.3 | 78.4 | 62.7 (54.9-70.4) |
| Score 8 | IDSA infection grade | 95.4 | 9.4 | 30.4 | 83.3 | 34.7 (27.0-42.3) |
| Score 9 | H/O Amputation | 47.7 | 94.3 | 77.8 | 81.3 | 80.7 (74.3-86.9) |
| Score 10 | Co-morbidity | 61.4 | 33.0 | 27.5 | 67.3 | 41.3 (33.4-49.2) |
| Score 11 | Ulcer depth grade | 97.7 | 14.1 | 32.1 | 93.8 | 38.7 (30.9-46.5) |
| Score 12 | H/O Bare feet walking | 52.3 | 50.9 | 30.7 | 72.0 | 51.3 (43.3-59.3) |
| Score 13 | Ankle brachial index | 50.0 | 78.3 | 48.9 | 79.0 | 70.0 [62.7-77.3] |
Distribution of sensitivity and specificity of total risk score (significant risk factors only) in predicting amputation.
| Total risk score | Sensitivity | Specificity | 1-specificity |
|---|
| 4.0 | 1.00 | 0.00 | 1.00 |
| 5.5 | 1.00 | 0.01 | 0.99 |
| 6.5 | 1.00 | 0.07 | 0.93 |
| 7.5 | 1.00 | 0.15 | 0.85 |
| 8.5 | 1.00 | 0.23 | 0.77 |
| 9.5 | 1.00 | 0.40 | 0.60 |
| 10.5 | 1.00 | 0.47 | 0.53 |
| 11.5 | 0.98 | 0.57 | 0.43 |
| 12.5 | 0.93 | 0.64 | 0.36 |
| 13.5 | 0.93 | 0.69 | 0.31 |
| 14.5 | 0.86 | 0.78 | 0.22 |
| 15.5 | 0.80 | 0.80 | 0.20 |
| 16.5 | 0.75 | 0.86 | 0.14 |
| 17.5 | 0.68 | 0.89 | 0.11 |
| 18.5 | 0.61 | 0.91 | 0.09 |
| 19.5 | 0.52 | 0.93 | 0.07 |
| 20.5 | 0.45 | 0.97 | 0.03 |
| 21.5 | 0.39 | 0.98 | 0.02 |
| 22.5 | 0.32 | 1.00 | 0.00 |
| 23.5 | 0.27 | 1.00 | 0.00 |
| 24.5 | 0.14 | 1.00 | 0.00 |
| 25.5 | 0.05 | 1.00 | 0.00 |
| 27.0 | 0.02 | 1.00 | 0.00 |
| 29.0 | 0.00 | 1.00 | 0.00 |
The smallest cut-off value is the minimum observed test value minus 1, and the largest cut-off value is the maximum observed test value plus 1. All the other cut-off values are the averages of two consecutive ordered observed test values.
The [Table/Fig-5] shows ROC curve for the total risk score including 13 risk factors (both significant and non-significant). The [Table/Fig-6] shows ROC curve for the total risk score (11 significant risk factors).
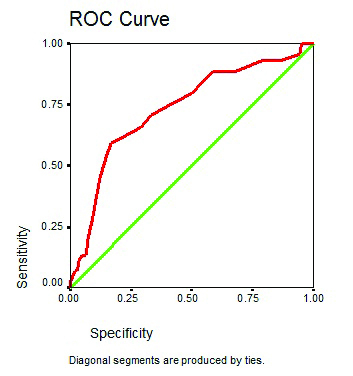
ROC curve for total risk score as a predictor amputation.
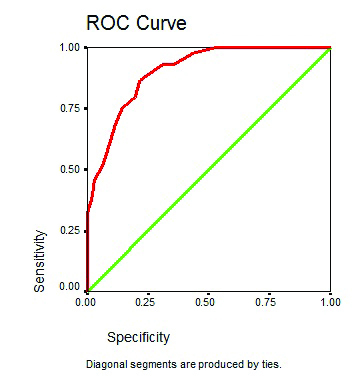
ROC curve for total risk score (significant risk factors only) as a predictor amputation.
Out of 150 cases studied, 118 cases (78.7%) did not require re-debridement or re-amputation, 23 cases (15.3%) required re-debridement and nine cases (6.0%) required re-amputation. Nine patients who underwent re-amputation, two cases (22.2%) had total risk score <10, four cases (44.4%) had risk score between 10.0-15.0 and three cases (33.3%) had total risk score more than 15.0. As depicted in [Table/Fig-7], the multivariate analysis revealed that duration of DM, HbA1c, Rutherford grading and ankle-brachial index were significantly associated with LEA (p-value<0.05).
Multivariate determinants of amputation (multivariate analysis using logistic regression analysis).
| Variables | Odds ratio (OR) | 95% CI of OR | p-value |
|---|
| Age group | <50 years | 1.0 | -- | -- |
| >50years | 1.77 | 0.86-2.36 | 0.103 |
| Duration of DM | <10 years | 1.0 | -- | -- |
| >10 years | 3.84 | 1.88-5.32 | 0.001 |
| Sensory neuropathy | Grade 0 | 1.0 | -- | -- |
| Grade ≥1 | 1.63 | 0.89-3.13 | 0.098 |
| Motor neuropathy | Grade 0 | 1.0 | -- | -- |
| Grade ≥1 | 1.83 | 0.90-2.46 | 0.229 |
| HbA1C | ≤7% | 1.0 | -- | -- |
| >7% | 5.84 | 3.03-7.49 | 0.001 |
| Rutherford grading | Grade 0 | 1.0 | -- | -- |
| Grade ≥1 | 3.48 | 2.06-5.23 | 0.002 |
| Deformity | Absent | 1.0 | -- | -- |
| Present | 1.41 | 0.83-2.32 | 0.343 |
| IDSA infection severity | Grade 1 | 1.0 | -- | -- |
| Grade ≥2 | 1.56 | 0.91-2.03 | 0.239 |
| H/O Amputation | Absent | 1.0 | -- | -- |
| Present | 1.53 | 0.91-1.96 | 0.314 |
| Co-morbidity | Absent | 1.0 | -- | -- |
| Present | 1.61 | 0.83-2.56 | 0.199 |
| Ulcer depth | Grade 1 | 1.0 | -- | -- |
| Grade ≥2 | 1.56 | 0.83-2.06 | 0.144 |
| H/O bare feet walking | Absent | 1.00 | -- | -- |
| Present | 1.45 | 0.84-1.94 | 0.203 |
| Ankle brachial index | 0.91-1.29 | 1.0 | -- | -- |
| ≤0.9 or ≥1.30 | 2.47 | 1.23-3.98 | 0.038 |
Discussion
Authors developed a risk score that can predict DM patient with high risk for LEA. It was observed that patients with the score of >16.5 were at increased risk of LEA and risk of amputation increased as the score increased. Multivariate analysis revealed that duration of DM, HbA1c, Rutherford grading and ankle-brachial index were significantly associated with LEA.
In the present study, 29.3% patients required LEA which is slightly higher than Lipsky BA et al., who reported that among 3,018 eligible patients, 21.4% underwent an LEA [25]. Lipsky BA et al., further reported that univariate analysis revealed that older age, peripheral vascular disease and previous LEA presence of a foot ulcer were significantly associated with LEA (p<0.05) [25]. Present authors also found that there was a strong correlation between previous history LEA in DM patients and present amputation. Majority of patients (77.8%) required amputation that had a history of LEA due to DM. The present research substantiated the finding of the study conducted by Lipsky BA et al., [25].
Adler AI et al., included 14 studies comprising 94,640 participants and 1,227 LEA cases [26]. Their review showed a strong association between the risk of LEA and increased levels of glycaemia in individuals with DM patients. The review further stated that there was a direct association between hyperglycaemia (as measured by HbA1c) and LEA according to which the risk of amputation raised 1.26 times for each percentage point increase in the HbA1c. The present study also showed a significant association between HbA1c levels and LEA, with a maximum number of amputations, occurred in 33/44 (75.0 %) patients having HbA1c ≥9.5 (80.5 mmol/mol).
Pickwell K et al., reported that deep ulcer (p<0.01), IDSA severity (p<0.01) were independent risk factors for LEA which was substantiated by the present research [27]. Increasing International Working Group on the Diabetic Foot (IWGDF) severity of infection also independently predicted amputation. They developed a risk score for any amputation and for amputations excluding the lesser toes (including the variables sex, pain on palpation, periwound oedema, ulcer size, ulcer depth, and peripheral arterial disease) that predicted amputation better than the IWGDF system (area under the ROC curves 0.80, 0.78, and 0.67, respectively). By way of comparison, the area under the ROC curve for the IWGDF system was 0.67 in their population.
In the present study, 68.1% of patients who underwent amputations had sensory neuropathy Grade 2 (≥3 sites). Adler AI et al., reported that sensory neuropathy due to DM is an independent risk factor for LEA [26]. Namgoong S et al., reported that the depth of the ulcer (p=0.001) and sensory neuropathy (p=0.023) were significantly associated with LEA [28].
Using the specificity and sensitivity of 13 risk factors we constructed a ROC curve according to which new risk score had a high prognostic accuracy based on the area under the curve of 0.733 which was higher than the IWGDF system which was 0.67 [27]. Out of the 13 risk factors, two risk factors were found to be not significantly associated with lower extremity amputation hence authors excluded them and the remaining 11 risk factors which had a significant correlation with diabetic foot amputation were used to construct another ROC curve which had a higher prognostic accuracy than the previous with area under the curve of 0.903. The resultant ROC curve yielded a cut-off score of 16.5.
The prospective study conducted by Adler AI et al., reported that peripheral sensory neuropathy, peripheral vascular disease, foot ulcers (particularly if they appear on the same side as the eventual LEA), former amputation, and treatment with insulin are independent risk factors for LEA in patients with DM [29].
Limitation
Limitations of the present study were authors have not studied the effect of risk factors on major and minor amputations separately. Also they have not included the history of previous lower extremity revascularisation procedure and studied the effects of venous insufficiency on diabetic foot ulcer.
Conclusion
Patients with a score of >16.5 were at increased risk of lower extremity amputation and risk of amputation increased as the score increased. This score may help clinicians identify patients at highest risk of LEA on examination. Once patient identification is achieved, methods to reduce the risk can be investigated. Multivariate analysis revealed that duration of DM, HbA1c, Rutherford grading and ankle-brachial index were significantly associated with LEA. Large multicentric studies with longer follow-up are required to confirm the efficacy of present risk score.
The smallest cut-off value is the minimum observed test value minus 1, and the largest cut-off value is the maximum observed test value plus 1. All the other cut-off values are the averages of two consecutive ordered observed test values.