Breast cancer is one of the commonest cancers in women in India with an estimated incidence of 155,000 new cases and 76,000 deaths in 2015 [1]. The risk factors for breast cancers include obesity (increased waist to hip ratio, increased BMI ≥30) and these are modifiable risk factors [2-4]. Breast cancer patients who are obese have been found to have a poorer prognosis as compared to those patients with normal BMI [5,6]. Some cohort studies have shown an association between different lifestyle factors like poor nutrition, obesity (excess body weight), and physical inactivity to breast cancer recurrence [5,7,8]. Additional studies have demonstrated lower breast cancer mortality to a higher quality of diet, reduced weight and increased physical activity [9-12]. Among the obese breast cancer patients, the ER-positive postmenopausal women seem to have poorer prognosis due to obesity as compared to others [13]. A recent study suggested that understanding and modifying these lifestyle risk factors along with the treatment for breast cancer can play a role in saving or prolonging the lives and also influence their quality of life [12,14]. The data on the influence of obesity on the outcome of breast cancer patients in Indian population is not available. The aim of the present study was to see if obesity influenced outcome of breast cancer patients in terms of DFS. Authors also intended to explore the relationship of obesity with age, stage at diagnosis, menopausal status, ER-positive status, recurrence of disease and diabetes as the comorbidity in the same cohort of patients.
Materials and Methods
This retrospective study was approved by the Institutional Review Board (registration number 10401 dated November 30, 2016). The data of a series of 341 female patients who were diagnosed and treated for invasive carcinoma breast in Christian Medical College, Vellore during the period of July 2010 and July 2015 were analysed. Data was collected from institutional medical records.
The inclusion criteria for this study were females aged 18 years and above who were diagnosed and treated for breast cancer between the above said duration. A total of 349 patients satisfied the inclusion criteria but eight patients were excluded due to lack of follow-up data.
BMI was calculated using the formula: patient’s weight divided by the height squared, weight in kilogram and height in meters. BMI ≥30 was considered obesity; BMI 25-29.9 was overweight and BMI <25 was normal weight as per WHO recommendations [15]. The outcome looked at was DFS which was the primary endpoint in this study. DFS was defined as the period from the date of diagnosis to the event (local or distant recurrence or death). The other variables analysed were age, menopausal status, stage at presentation, ER-status, first recurrence or progression at follow-up and time to recurrence or progression and BMI.
Statistical Analysis
DFS rates were estimated using the Kaplan-Meier method. The Fisher-Exact test was used for various clinical variables to determine if BMI influenced them. Unadjusted Cox proportional hazard models were used to determine if BMI influenced outcome. The p-value less than 0.05 was considered as significant. The analysis was done using SPSS version 16.0 and Stata 15.0.
Results
A follow-up period of the median of 37 months (range: 3-82) for 341 women with breast cancer, 24% (n=82) developed metastasis or progression. Five-year estimation of DFS was 70.7% (95%, CI 0.64-0.76) for the total study population.
In this cohort, 46% of patients were between 36-50 years of age and 43.1% were more than 50-years of age. Median age was 50 years (23-73). Mean height of the cohort was 152.71 cm (±5.95). Mean weight of the cohort was 60.4 kg (±11.6). Mean BMI of the cohort was 25.8(±4.62). Median BMI was 25.7 (15.6-46). Obesity was seen in 15.5% (n=53), overweight in 39.6% (n=135), normal weight in 40.2% (n=137) and underweight in 4.7% (n=16). Among obese patients, WHO Class I obesity (BMI 30.0-34.9) was the majority 81.1% as compared to class II and class III as shown in [Table/Fig-1]. The patient characteristics with regard to BMI are summarised in [Table/Fig-1].
Patient characteristics with regard to weight.
| Characteristic | Percentage(n) |
|---|
| BMI (kg/m2) | |
| Obese (BMI ≥30) | 15.5 (53) |
| WHO class I obesity (BMI 30-34.9) | 43 (81.1%) |
| WHO class II obesity (BMI 35-39.9) | 7 (13.2%) |
| WHO class III obesity (BMI ≥40) | 3 (5.7%) |
| Overweight (BMI 25-29.9) | 39.6 (135) |
| Normal (BMI <25) | 40.2 (137) |
| Underweight (BMI ≤18.5) | 4.7 (16) |
BMI: Body mass index
Distribution of overweight and obese patients to various clinical variables.
| Variables | All(n=341) | BMI<25 (n=153) | BMI ≥25 (n=188) | (p-value) |
|---|
| Age (years) |
| ≤35 | 37 (10.8%) | 22 (59.5%) | 15 (40.5%) | 0.175 |
| 36-50 | 157 (46.0%) | 68 (43.3%) | 89 (56.7%) |
| >50 | 147 (43.2%) | 63 (42.9%) | 84 (57.1%) |
| Menopausal status |
| Premenopausal | 155 (45.5%) | 76 (49%) | 79 (51%) | 0.158 |
| Postmenopausal | 186 (54.5%) | 77 (41.4%) | 109 (59.6%) |
| Stage at diagnosis |
| Early breast cancer | 59 (17.3%) | 30 (50.8%) | 29 (49.2%) | 0.568 |
| Locally advanced | 255 (74.8%) | 112 (43.9%) | 143 (56.1%) |
| Metastatic | 27 (7.9%) | 11 (40.7%) | 16 (59.3%) |
| ER status |
| Positive | 182 (53.4%) | 75 (41.2%) | 107 (58.8%) | 0.146 |
| Negative | 159 (46.6%) | 78 (49.1%) | 81 (50.9%) |
| Triple negative breast cancer |
| Yes | 70 (20.5%) | 31 (44.3%) | 39 (55.7%) | 0.193 |
| No | 271 (79.5%) | 122 (45%) | 149 (55%) |
| Diabetes mellitus |
| Yes | 67 (19.6%) | 24 (35.8%) | 43 (64.2%) | 0.097 |
| No | 274 (80.4%) | 129 (47%) | 145 (53%) |
| Recurrence/Progression |
| Yes | 82 (24%) | 40 (48.8%) | 42 (51.2%) | 0.414 |
| No | 259 (76%) | 113 (43.6%) | 146 (56.4%) |
BMI: Body mass index; Statistical Test-Pearson Chi-Square Test
As shown in [Table/Fig-2], BMI ≥25 was more prevalent in age group >35 years as compared to age group ≤35 years (59.5% Vs. 40.5%); Prevalence of overweight (BMI ≥25) was more in women with locally advanced disease (56.1%) as compared to early disease (49.2%). Though not statistically significant, BMI ≥25 was prevalent in postmenopausal women, ER-positive, triple negative and diabetic women.
Recurrence
Mean duration of follow-up was 34.06 months with a median of 37 months (3-82). Disease recurrence or progression was seen in 24% of the cohort and 51.2% of them who recurred or progressed had BMI ≥ 25.
Local recurrences: Local recurrences are shown in [Table/Fig-3]. There was only one Ipsilateral Breast Tumour Recurrence (IBTR). Two of the nine chest wall recurrences were prior to Radition Therapy (RT) (one while on adjuvant chemo, one had chosen not to receive RT).
Pattern of recurrence or progression.
| Stage at diagnosis | Recurrences |
|---|
| EBC | LABC | MBC |
|---|
| Number of patients in whom recurrence occured | 5 | 65 | 12 |
| Total Number of Patients | 59 | 255 | 27 |
| Recurrence in Percentage | 8.47 | 25.5 | 44.4 |
| Chest wall recurrence | 0 | 9 | 0 |
| Ipsilateral Breast Tumour Recurrence (IBTR) | 1 | 0 | 0 |
| Contralateral breast | 0 | 4 | 1 |
| Nodal recurrence | 1 | 4 | 1 |
| Distant metastases | 3 | 52 | 11 |
| Both local and distant | 0 | 9 | 1 |
EBC: Early breast cancer; LABC: Locally advanced breast cancer; MBC: Metastatic breast cancer
As shown in [Table/Fig-4], the predictors of DFS such as age, menopausal status, stage at diagnosis, receptor status, diabetes, overweight and obesity were examined by univariate analysis. ER-status (HR 1.68(1.09-2.60) p-value 0.02), locally advanced breast cancer {HR 3.56 (1.43-8.83); p-value 0.006} and metastatic breast cancer {8.19 (2.88-23.29); p-value-<0.001} were found to be significant for DFS. Overweight and obesity were not found to be significant factors influencing DFS. Among the patients with WHO Class II and III obesity (n=10), 3 (30%) of them had recurrence or progression. Two of them had locally advanced disease at diagnosis and one patient had metastatic disease at diagnosis.
Univariate analysis of clinicopathologic variables.
| Variables | No of events†/n | HR (95%, CI) | p-value |
|---|
| Age (years) |
| ≤35 | 11/37 | | |
| 36-50 | 42/157 | 0.87 (0.45-1.71) | 0.702 |
| >50 | 29/147 | 0.62 (0.31-1.24) | 0.178 |
| Menopause |
| Premenopause | 44/155 | | |
| Postmenopause | 38/186 | 0.7 (0.45-1.08) | 0.112 |
| Stage at diagnosis |
| Early breast cancer | 5/59 | | |
| Locally advanced | 65/255 | 3.56 (1.43-8.83) | 0.006* |
| Metastatic | 12/27 | 8.19 (2.88-23.29) | <0.001* |
| ER-status |
| Positive | 37/182 | | |
| Negative | 45/159 | 1.68 (1.09-2.60) | 0.020* |
| Triple negative |
| Yes | 19/70 | 1.42 (0.85-2.38) | 0.175 |
| No | 63/271 | | |
| Diabetes |
| Yes | 15/67 | | |
| No | 67/273 | 0.94 (0.54-1.65) | 0.835 |
| Weight |
| Normal (BMI <25) | 40/153 | | |
| Abnormal (BMI ≥25) | 42/188 | 0.76 (0.49-1.18) | 0.220 |
| Obesity |
| Obese (BMI ≥30) | 14/53 | 1.03 (0.58-1.83) | 0.917 |
| Nonobese (BMI <30) | 68/288 | | |
| BMI |
| Obese (BMI≥ 30) | 14/53 | 0.88 (0.48-1.63) | 0.698 |
| Overweight (25-29.9) | 28/135 | 0.71 (0.44-1.16) | 0.170 |
| Normal (BMI <25) | 40/153 | | |
*Significant p-value; †event=recurrence or progression; BMI: Body mass index; statistical test: Cox proportional hazard model
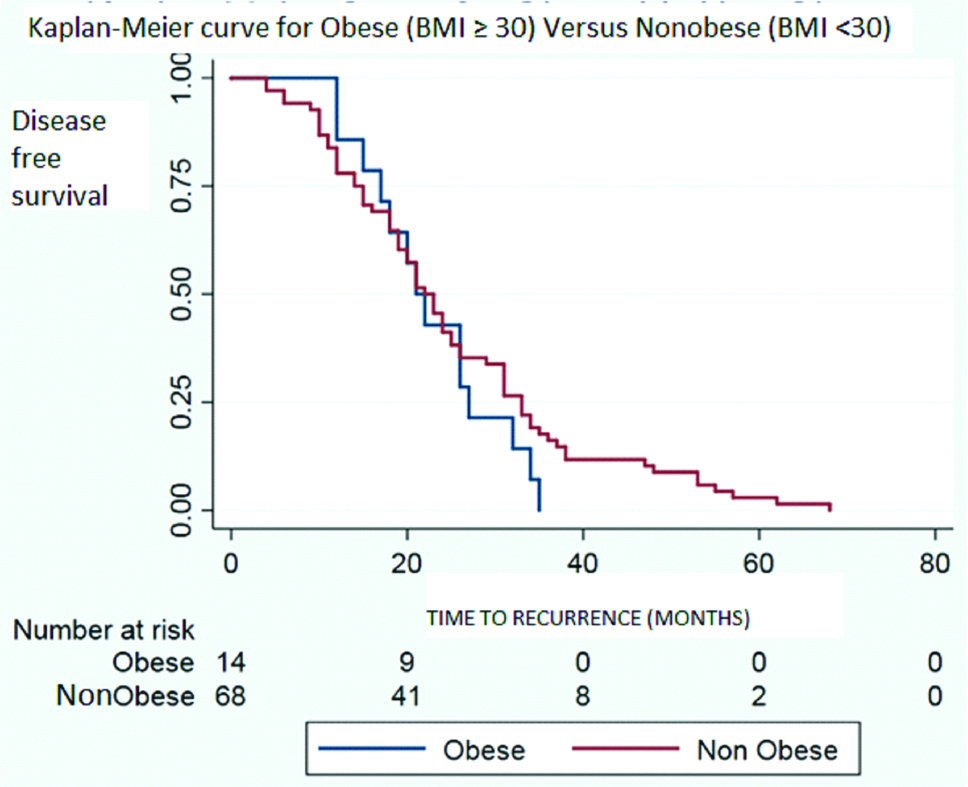
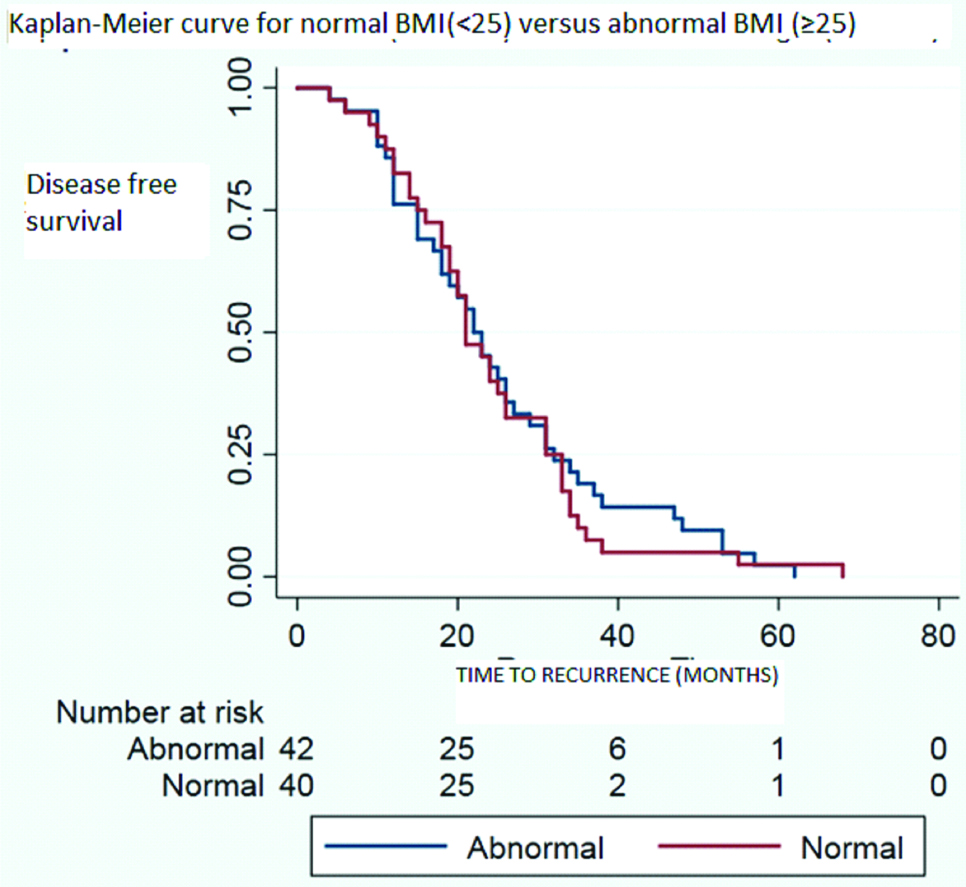
Time of recurrence has been compared between obese and non-obese population of the cohort in whom recurrence or progression occured in [Table/Fig-5] and the same has been compared between normal and abnormal BMI population of the cohort whom recurrence or progression occured in [Table/Fig-6]. Both the results shows no significant difference. Median time to recurrence was 21.5 months for obese group (BMI ≥30); 23 months for overweight group (25-29.9) and 21 months for normal weight group (BMI <25).
Time of recurrence for obese (BMI ≥30) versus non-obese (BMI <30) population in the cohort who recurred or progressed.
Time of recurrence for normal (BMI <25) versus abnormal BMI (≥25) population in the cohort who recurred or progressed.
Discussion
Overweight or obesity is studied to establish its role on incidence, recurrence and prognosis of breast cancer. In the recent meta-analysis, Chan DS et al., pooled in data from 82 studies and showed that obesity at diagnosis of breast cancer increased the mortality by 35% [7]. Zhang M et al., demonstrated that obesity affected late outcomes too in breast cancer [16]. Al Jarroudi O et al., showed that triple negative breast cancer outcomes are influenced by obesity [17]. Alternatively, Mei L et al., could not demonstrate the same [18]. In SUCCESS trial, Widschwendter P et al., reported DFS with a Hazard Ratio (HR) of 1.06 (0.81-1.4) in the slightly obese group. In this study, DFS for obese patients had HR of 0.88 (0.48-1.63) [11]. [Table/Fig-7] lists the recently published literature that reported on obesity influencing recurrence in breast cancer [8,11,16-20].
Comparison of Hazard ratio for recurrence in obesity with recent published literature.
| Study | HR (95%, CI) for recurrence | Specifics of study population |
|---|
| Current study | 0.88 (0.48-1.63) | BMI ≥30 |
| Cespedes Feliciano EM et al., [8] | 1.02 (0.67-1.54) | BMI 30 to 35 |
| Widschwendter P et al., [11] | 1.06 (0.81-1.4) | BMI 30 to 35 |
| Zhang M et al., [16] | 1.01 (0.72-1.42) | BMI ≥26.3 |
| Al Jarroudi O et al., [17] | 1.89 (1.05-3.4) | TNBC |
| Mei L et al., [18] | 0.93 (0.74-1.17) | TNBC |
| Maliniak ML et al., [19] | 1.27 (1.14-1.41) | ≥65 years, breast cancer specific mortality |
| Bergom C et al., [20] | 1.09 (0.98-1.19) | Locoregional recurrence free interval |
HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; TNBC: Triple negative breast cancer
ER Positivity and Obesity
Cespedes Feliciano EM et al., demonstrated that the outcome of luminal A cancers was adversely affected by obesity [8]. Sparano AJ et al., demonstrated inferior outcomes in ER-positive breast cancers with obesity [9]. DFS was lower in obese breast cancer patients with an HR of 1.24 with 95% Confidence Interval (CI) of 1.06-1.46 and overall survival was also lower with an HR of 1.37 with 95% CI of 1.13-1.67. In this study, DFS in ER-positive women with BMI ≥25 was higher compared to ER negative women with BMI ≥25 with an HR 1.68 (1.09-2.60).
Menopausal Status and Obesity
Study demonstrated an increase in the risk of breast cancer in obese postmenopausal women [21]. Chan DS et al., also demonstrated that obesity in breast cancer increased mortality in premenopausal women (relative risk RR of 1.75) in comparison to those in postmenopausal women (RR of 1.34) [7]. Though postmenopausal women are obese compared to premenopausal women, premenopausal women with obesity have poorer outcomes in breast cancer. In this study, premenopausal women had increased DFS compared with postmenopausal women with an HR 0.7 (0.45-1.08).
Most of the studies as shown in the [Table/Fig-7] could demonstrate worse outcomes in breast cancer women due to obesity except for Mei L et al., and including the present study. Overall, overweight does not seem to affect the outcome but obesity with BMI >35 seems to definitely affect outcomes in breast cancer.
Molecular Basis
To explain how obesity influences breast cancer at the molecular level, Ligibel JA et al., lists the mechanisms for increased risk of breast cancer and worst outcome in obese or overweight women [22]. These include high endogenous oestrogen levels in these women, high insulin levels being similar to Insulin like Growth Factor (IGF) -1 inhibits signalling pathways with IGF-1, imbalance of adipokines. Leptin that has antiapoptotic activity are more and adiponectin that is proapoptotic are less [23].
Proportion of Obesity among Women with Breast Cancer
Proportion of abnormal BMI to normal BMI subjects were almost equal in this study (55% versus 45%) in contrast to published literature wherein the proportion of abnormal weight subjects to normal weight subjects was 90% versus 10% according to Bergom C et al., and 62% versus 38% according to Choi Y et al., Whether overweight and obesity has not yet become a significant problem in breast cancer among Indian women is something to be answered [20,24]. Although, there have been studies proving obesity increasing incidence of breast cancer in Indian women as compared to that of women of normal weight, obesity influencing outcome of breast cancer patients is still unclear [3].
Severity of Obesity
There are studies that did not find BMI <40 affecting breast cancer mortality concluding that severe obesity alone affects breast cancer outcomes [25]. Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial subjects were relooked to see if BMI affected their outcome and it did show that BMI >35 increased the risk of recurrence by 60% [26]. Austrian breast cancers study group 12 (ABCSG-12) also showed similar results among its participants [27]. WHO Class II obesity (BMI >35) was seen only in 2.9% in this cohort.
Limitation
In this study, Disease Free Survival (DFS) was not affected by obesity or overweight. The reasons could be that the sample size was too small to detect the difference (Class II and above obesity patients were less in number in this cohort); BMI alone was taken into consideration to define obesity; BMI at diagnosis only was considered and was not monitored throughout treatment and follow-up; the outcome studied was DFS and not overall survival; follow-up was shorter; and that breast cancer was heterogenous in nature; this cohort included all types and this was retrospective study in nature.
Future recommendations would be to test if all lifestyle measures including weight reduction to aim normal BMI, physical activity, and healthy balanced diet, social and spiritual healthy lifestyle would influence the outcome of locally advanced breast cancer patients.
Conclusion
There was no statistically significant association found between obesity versus DFS, age, menopausal status, stage at diagnosis, ER-status, or diabetes among breast cancer patients in this study. However, there was a higher incidence of overweight and obesity noted in the breast cancer patients especially in postmenopausal women, ER-positive disease and those with later stages at presentation. Randomised prospective controlled studies in Indian population are needed to further validate these findings and look at the influence obesity has on breast cancer incidence and outcome.
BMI: Body mass index
BMI: Body mass index; Statistical Test-Pearson Chi-Square Test
HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; TNBC: Triple negative breast cancer