Diabetes Mellitus (DM) is defined as the set of metabolic disorders characterised by hyperglycaemia resulting from defects in insulin action, insulin secretion or both [1]. Chronic diabetes is associated with damage, dysfunction, and failure of different organs, especially the nerves, heart, eyes, kidneys, and blood vessels [2]. Long-term diabetes has been associated with microvascular complications such as nephropathy, retinopathy, neuropathy. Also, the macrovascular complications include cardiomyopathy, vasculopathy, dermatopathy and atherosclerosis [3].
The primary microvascular complications of diabetes lead to kidney damage known as Diabetic Nephropathy (DN), the most common complication of Type 2 Diabetes Mellitus [4]. DN is characterised by a set of functional kidney abnormalities in diabetes patients [5].
Diabetic nephropathy develops in more than 40% of patients with diabetes, when elevated glucose levels are maintained for a long duration of time [6]. This observation raised the notion that a subset of patients has an increased susceptibility to diabetic nephropathy. The cystatin C protein has a molecular mass of (13.359 KDa) and consists of 120 amino acid residues in a single polypeptide chain with two disulfide bonds [7-9]. Cystatin C is a protein inhibitor of cysteine proteinases. This is synthesised at a constant rate by all nucleated cells. Because, it has low molecular weight and high isoelectric point, it can be eliminated exclusively by glomerular filtration [8].
Cystatin C concentration is not influenced by sex or protein ingestion. However, it is sensitive to small changes in glomerular filtration [10,11]. Because of these characteristics, cystatin C could serve as an ideal marker of the glomerular filtration status. The aim of the study was to evaluate the clinical advantage of serum cystatin C in predicting renal impairment in patients with Type 2 Diabetes Mellitus when compared to serum creatinine.
Materials and Methods
The present cross-sectional study was carried out on 170 participants out of which 85 were patients diagnosed with diabetic nephropathy (Serum Creatinine level >1.5 mg/dL) and the other 85 were diabetics without nephropathy (Serum Creatinine level <1.3 mg/dL in male and <1.0 mg/dL in female) [12] serving as control. The participants of both gender and age >45 years who were enrolled to the Shree Krishna hospital Karamsad-Anand-Gujarat India, during the period from September 2015 to April 2017, were included. Patients with known non-diabetic renal disease and patients with Type 1 diabetes mellitus were excluded. Blood samples were collected according to the protocol used in the laboratory of Biochemistry in Shree Krishna Hospital. All participants provided informed consent.
The blood samples were collected in three tubes:
1) Plain tube for creatinine, urea and cystatin C
2) Sodium fluoride for Fasting Plasma Glucose
3) Ethylenediaminetetraacetic Acid for HbA1c
The study was approved by the Human Research Ethics Committee (HREC) of Pramukhswami Medical College (PSMC), Karamsad. No. 2015/387115).
Biochemical tests included Plasma Fasting Blood Glucose, Random Blood Glucose, Post Prandial Blood Glucose, Serum Urea, Creatinine, Glycated haemoglobin (HbA1c). These were estimated by using Automated Chemistry Analyser (Siemens Dimensions RxL/Xpand).
Fasting Serum Lipid Profile (Cholesterol, Triglycerides, HDL and LDL Cholesterol) were estimated using Chemistry Automated Analyser (Siemens Dimensions RxL/Xpand). The LDL-Cholesterol was calculated using Friedewald formula [12,13].
Serum samples were collected to measure cystatin C using immunoturbidimetric assay (On TURBODYNE TM SC). For serum cystatin C, two levels of human-based control serum were analysed with each run. Other serum parameters (glucose, urea and creatinine) used two levels of lyophilised multi-control sera, normal and abnormal levels daily.
The following methods were used to perform various biochemical parameters: Plasma Glucose was measured by Hexokinase method, Serum Creatinine was measured by Jaffe’s method, Serum Urea was measured by using Urease and Glycated Haemoglobin Hb (HbA1c) was determined by Turbidimetric Inhibition Immunoassay Method [14]. Glomerular Filtration Rate (GFR) calculated according to kidney disease epidemiology collaboration equation (CKD-EPI) creatinine and cystatin formula:
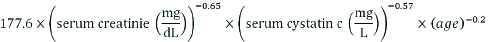
The Modification of Diet in Renal Disease equation (MDRD) formula and CKD-EPI formulas are already standardised for a 1.73 m2 body surface area. The correction factor of 0.742 was used for women [15-17].
Classification stage of renal failure was according to the value of GFR. (according to The National Kidney Foundation classification) [18].
Statistical Analysis
The results were presented as mean±standard deviation (SD). The statistical significance between the groups was analysed by studying t-test and correlation test between various parameters. The value of p<0.05 was assessed as significant. The data were computed and analysed using Statistical Packages for Social Science (SPSS 20. USA). EndnoteX7 was used for references management.
Results
A total of 170 participants were divided into 85 patients with diabetic nephropathy {55 males (64.7%) and 30 females (35.3%)} and 85 patients with diabetes without nephropathy {60 males (70.6%) and 25 females (29.4%)} [Table/Fig-1].
Distribution of study population by gender and type of diabetes.
| Variables | Diabetic types | Total |
|---|
| Diabetics without nephropathy | Diabetics nephropathy |
|---|
| Gender | M | Count | 60 | 55 | 115 |
| % of Total | 35.3% | 32.4% | 67.6% |
| F | Count | 25 | 30 | 55 |
| % of Total | 14.7% | 17.6% | 32.4% |
| Total | Count | 85 | 85 | 170 |
| % of Total | 50.0% | 50.0% | 100.0% |
The results showed that the mean cystatin C level was higher in diabetics with nephropathy (1.87±0.51 mg/L) than diabetics without nephropathy (1.025±0.30 mg/L), and the difference was significant with p-value <0.001 [Table/Fig-2].
Serum cystatin C level in diabetics with nephropathy and diabetics without nephropathy patients.
| Serum parameter | Diabetic without nephropathy | Diabetic with nephropathy | Statistics |
|---|
| Males Mean±SD | Females Mean±SD | Males Mean±SD | Females Mean±SD | p-value |
|---|
| Cystatin C (mg/L) | 1.03±0.36 | 1.02±0.25 | 1.84±0.49 | 1.9±0.53 | <0.0001 |
p-value by independent samples t-test, p<0.01 is statistical significant. High significant with cystatin C
The results also showed that the level of serum cystatin was higher in some patients ((32.9%) 28 out of 85 cases) with a normal level of creatinine. But the level of serum cystatin C was abnormally high in all the patients who had an abnormally high serum creatinine level. This was significant with p<0.001 [Table/Fig-3].
Comparison between the serum creatinine and cystatin C in diabetics with nephropathy (abnormal creatinine >1.5 mg/dL) and diabetics without nephropathy (normal creatinine male <1.3 mg/dL, female <1.0 mg/dL) [12].
| Cystatin C | Total | p-value |
|---|
| Normal (<1.16 mg/L) | Higher (>1.16 mg/L) |
|---|
| Creatinine | Normal (Male<1.3 mg/dL, Female <1.0 mg/dL) (DM without nephropathy) | 57 | 28 | 85 | p<0.001 |
| Abnormal (>1.5 mg/dL) (DM with nephropathy) | 0 | 85 | 85 |
| Total | 57 | 113 | 170 |
p-value by independent samples t-test, p<0.01 is statistical significant
The relationship of serum cystatin C with creatinine is of positive correlation (r=0.55, p<0.0001) [Table/Fig-4].
Correlation of serum cystatin C with creatinine and urea.
| Parameters | Correlation coefficient r | Significance level |
|---|
| Cystatin C with Creatinine | 0.55 | p<0.001 |
| Cystatin C with urea | 0.66 | p<0.001 |
**Correlation is significant at the 0.01 level (2-tailed)
The results showed the relationship of serum cystatin C with creatinine, urea in diabetic nephropathy and diabetics without nephropathy. Positive Pearson’s correlation between cystatin C and creatinine, urea levels, were significant with (r=0.55; ‘p’<0.001 and r=0.66; ‘p’<0.001) respectively.
[Table/Fig-5] The results showed a correlation of serum cystatin C, serum creatinine with diabetes. The Pearson correlation test showed a strong positive association with cystatin C (r=0.710 and ‘p’<0.0001) versus lower personal correlation with creatinine (r=0.62 and p<0.0001). Cystatin C had strong positive correlation more than creatinine. (p-value <0.0001)
Correlation of serum cystatin C, creatinine with diabetic with and without nephropathy.
| Cystatin C | Creatinine |
|---|
| Diabetic types | Pearson Correlation | 0.710** | 0.626** |
| Sig. (2-tailed) | 0.0001 | 0.0001 |
| n | 170 | 170 |
**Correlation is significant at the 0.01 level (2-tailed)
[Table/Fig-6] shows that out of total patients who had diabetes without nephropathy the study found 27 cases with moderate decrease of GFR and 41 patients had mild decrease of GFR, but only 17 patients had normal GFR. In diabetics with nephropathy we found 55 cases with moderate decrease of GFR, 28 patients had severe decrease of GFR and 2 patients had Kidney failure with GFR <15 mL/min/1.73 m2.
The distribution of the cases according to the stages of renal disease dependent on estimating GFR using serum cystatin C in diabetics with and without nephropathy.
| Diabetic types | Total No: of cases |
|---|
| Diabetic without nephropathy No: of cases | Diabetics with nephropathy No: of cases |
|---|
| DN. Stages | G1. NormalGFR >90 mL/min/1.73 m2 | 17 | 0 | 17 |
| G2. Mild decreaseGFR=60-89 mL/min/1.73 m2 | 41 | 0 | 41 |
| G3. Moderate decreaseGFR=30-59 mL/min/1.73 m2 | 27 | 55 | 82 |
| G4. Severe decreaseGFR=15-29” mL/min/1.73 m2 | 0 | 28 | 28 |
| G5. Kidney failureGFR <15 mL/min/1.73 m2 | 0 | 2 | 2 |
| Total of cases | 85 | 85 | 170 |
According to estimating GFR using serum creatinine, out of total patients who had diabetes with nephropathy, the study found 58 patients in stage three (moderate decrease of GFR), 25 patients with stage four (severe decrease of GFR) and 2 patients with stage five (Kidney failure with GFR <15 mL/min/1.73 m2.) [Table/Fig-7].
The distribution of the cases according to the stages of renal disease dependent on estimating GFR using serum creatinine in diabetics with and without nephropathy.
| Diabetic types | Total No: of cases |
|---|
| Diabetic without nephropathy No: of cases | Diabetics with nephropathy No: of cases |
|---|
| DN. Stages | G1. Normal >90GFR >90 mL/min/1.73 m2 | 32 | 0 | 32 |
| G2. Mild decreaseGFR=60-89 mL/min/1.73 m2 | 53 | 0 | 53 |
| G3. Moderate decreaseGFR=30-59 mL/min/1.73 m2 | 0 | 58 | 58 |
| G4. Severe decreaseGFR=15-29” mL/min/1.73 m2 | 0 | 25 | 25 |
| G5. Kidney failureGFR <15 mL/min/1.73 m2 | 0 | 2 | 2 |
| Total of cases | 85 | 85 | 170 |
From [Table/Fig-6,7], we found cystatin C is the best marker of creatinine for GFR estimation.
Sensitivity and Specificity of Creatinine and Cystatin C
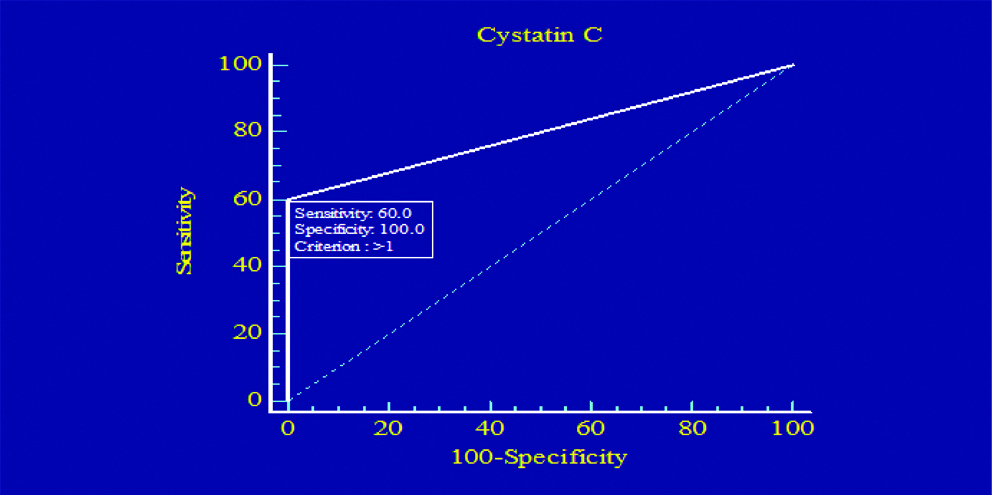
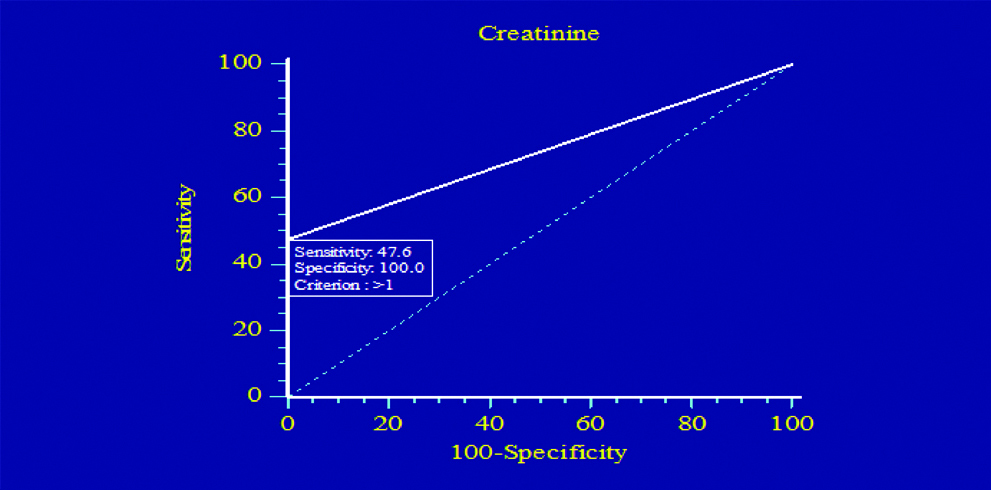
[Table/Fig-8,9] Illustrates the ROC plots to assess the diagnostic efficiency of serum cystatin C and serum creatinine. This analysis for all patients included in the study showed that serum cystatin C had higher sensitivity diagnostic accuracy than serum creatinine in diagnosis of DN. The sensitivity was 60% for serum cystatin C, and 47.6% for serum creatinine.
Sensitivity and specificity of cystatin C.
Sensitivity and specificity of creatinine.
Discussion
Diabetes is characterised by sustained hyperglycaemia, which causes changes in metabolic and haemodynamic properties [19]. Renal enlargement is one of the key features occurring during initial changes of diabetes [20]. Diabetes Mellitus is a metabolic disorder and major health problem of all the countries. Low and middle-income countries face the greatest burden of the disease. The total number of people with diabetes is projected to rise from 171 million in the year 2000 to 200 million in the year 2013 and to 552 million in the year 2030 [21]. Type 2 Diabetes Mellitus is the common type of diabetes. It usually begins in middle age due to poor secretion of insulin and/or insulin resistance in target cells. One of the most important clinical features of diabetes is the harmful effects on the kidneys that may develop into DN [2,22,23]. Diabetic nephropathy occurs in approximately one-third of individuals with type-1 and type-2 DM. It is associated with high rates of morbidity and mortality [24]. There is widespread agreement that specific tests are necessary to monitor early signs of diabetic nephropathy. Previous studies have been carried out on microalbuminuria and other early markers for diabetic nephropathy among Type 2 Diabetes Mellitus patients [25,26].
It is very important in the management of diabetes mellitus to detect diabetic nephropathy as early as possible and to prevent its development. In this study, the aim was to assess the levels of serum cystatin C in a group of patients with Type 2 Diabetes Mellitus by categorising them into two groups depending on kidney dysfunction (nephropathy and without nephropathy). Data presented in this study, the mean age of males with diabetic nephropathy was 68.1 years and females 64.3 years. Other studies suggest that Type 2 Diabetes Mellitus usually develops after age 40 [27,28]. Evaluation of renal function is important in diabetic patients and indicators are needed to determine structural and functional changes early in diabetic nephropathy. Serum creatinine is a specific test but not very sensitive because its levels do not increase significantly until the GFR is reduced to less than 50% [29]. In addition, serum creatinine concentration is significantly affected by many factors: age, sex, muscle mass, dietary intake, changes in tubular secretion and various drugs as well as endogenous substances that interfere with its assay. However, serum cystatin C may not be affected by these factors to a significant degree; this could explain the possible superiority of serum cystatin C over serum creatinine for predicting GFR. [30,31]. Cystatin C was widely reported to be independent and unaffected by sex, age, height, weight and muscle mass [32].
In this study, the cystatin C, unlike creatinine, was not affected by sex, but there was a weak positive correlation with age. The cystatin C level was increased with increase in the age. These effects, indicating that serum cystatin C positive correlation with age, it is significant with p-value=0.005 and also significant with creatinine, p-value=0.003. In some previous reports, there is a discrepancy in the relationship between age and serum cystatin C [33,34]. Although, other studies have shown that cystatin C levels increase with age [35].
A recent study [9], reported the result of GFR. It used cystatin C compared with the use of creatinine. Higher correlation coefficients with cystatin C (r=0.7065) compared with creatinine use (r=0.4767) with p=0.001. In this study, cystatin C level showed a strong positive correlation for diabetics with and without nephropathy compared with creatinine (0.71 or. 0.62; p<0.001).
In addition, the positive correlation between serum cystatin C and each of serum urea and creatinine suggesting that serum cystatin C better than serum urea and creatinine. Similar findings were observed in the previous studies done by El Telbani RM and Tian S, Kusano E et al., [36,37]. The results show that cystatin C is an early marker in diabetes more accurate than creatinine, especially in patients with diabetic nephropathy, it is found that 28 of the total 85 patients with diabetes had a high cystatin C index unlike creatinine in the normal range. This means that cystatin C is more sensitive than creatinine in the diagnosis of early renal failure. Waheed HJ et al., showed that cystatin C was a good marker of impaired renal function and its more sensitive marker. Cystatin C had a good correlation with creatinine [38]. Also, Shetty V et al., reported that the serum cystatin C is independent marker of incipient diabetic nephropathy in Type 2 diabetic patients [39].
These results are different to that resulted by Oddoze C et al., they found that the correlation coefficients with GFR were –0.77 for serum creatinine level and -0.65 for serum cystatin C level (all p<0.001). Their study also concluded that the serum cystatin C is not better than serum creatinine levels for estimating GFR in patients with diabetes [40].
Limitation
The study was limited in sample size and time duration. Complete evaluation of the clinical applicability of cystatin C will require a larger sample size and to follow the disease status over a sufficient period to detect changes in renal function (measurement of cystatin C during the pathological stages of the kidney and compare with the results of creatinine and creatinine clearance results).
Conclusion
The serum cystatin C is found to be more sensitive than creatinine in diagnostic accuracy to distinguish patients with nephropathy. Cystatin C is a useful marker for early detection of diabetic nephropathy. The results of this study suggest that cystatin C measurement in serum is useful for the early detection of renal impairment, is better than serum creatinine in the patients suffering from diabetes.
p-value by independent samples t-test, p<0.01 is statistical significant. High significant with cystatin C
p-value by independent samples t-test, p<0.01 is statistical significant
**Correlation is significant at the 0.01 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)