Hypertension is a trait opposed to a specific disease and with an ongoing global increase in the incidence. Hypertension is classified into two groups, primary or essential hypertension, and secondary hypertension. Primary hypertension is defined as a rise in blood pressure of unknown cause and secondary is caused by disease of kidney, endocrines or some other organs. An individual with hypertension is at increased risk factor for stroke, heart disease, and kidney failure [1].
Oxidative stress is defined when there is an imbalance between the reactive oxygen species generation and the antioxidant defence system. Increased vascular oxidative stress could be involved in the pathogenesis of hypertension. Lipid peroxidation is a chain reaction initiated by free radicals which provides a continuous supply of other free radicals formed from unsaturated fatty acids which further initiate peroxidation. Malondialdehyde, the end product of lipid per-oxidation can be used as a marker for lipid peroxidation and oxidative stress.
Paraoxonase 1 (PON1), an enzyme which is found exclusively associated with HDL in serum. It protects LDL from oxidation. In humans, PON 1 is an independent, genetic risk factor for coronary artery disease [2,3] and low PON1 activities are observed in atherosclerotic and hypercholesterolemic patients [2,4-6].
An important role is played by PON 1 in the antioxidant system as it is thought to degrade oxidised phospholipids in lipoproteins [7]. In the diseases involving oxidative stress, the circulating PON 1 levels have been found to be altered [8].
There are various studies that point that the oxidative stress is increased in hypertensive patients and it may be caused due to destruction of endothelial cells that are responsible for arterial relaxation. Superoxide anion acts as a vasoconstrictor and is a major determinant of Nitric Oxide (NO) biosynthesis and bioavailability; therefore, it can modify the endothelial cell function. In humans, hypertension is associated with a decrease in NO bioavailability and an increase in oxidative stress [9]. Decreased catalase and/or SOD antioxidant activity along with reduced levels of Reactive Oxygen Species (ROS) scavengers such as glutathione and vitamins C and E also contribute to oxidative stress [10,11].
The study aimed at finding out the concentrations of PON 1 along with oxidative stress markers in hypertensive dyslipidemic patients and ultimately looking for correlation between the two.
Materials and Methods
This cross-sectional study was carried out in the Department of Biochemistry, Santosh Medical College and Hospital, Ghaziabad in collaboration with Department of Medicine and Department of Biochemistry, Muzaffarnagar Medical College and Hospital, Muzaffarnagar, after getting approval from ethical committee of Santosh and Muzaffarnagar Medical College and informed consent was taken from all the subjects. The patients were selected from outpatient department of medicine, Muzaffarnagar Medical College, on the basis of inclusion and exclusion criteria. The prevalence rate of hypertension in Muzaffarnagar region was around 20% accordingly, the minimum sample size has been calculated using the appropriate sample size formula:
n=z2pq/d2
Where z=1.96 at 95 % confidence interval,
p=0.20 and q=1-p=0.80,
d=absolute error 5%
n= (1.96)2×0.20×0.80/(0.05)2
=245.86≈246
Hence, the minimum sample size for cases=246
Inclusion criteria: Five hundred male subjects were included in this study aged between 30-60 years out of which 250 were hypertensive dyslipidemic and rest 250 were normal healthy controls. Control group included normotensive subjects with a blood pressure of 110-139/60-89 mm Hg while hypertensive case included those with a blood pressure of ≥120/90 mm Hg.
Exclusion criteria: Patients with diabetes, asthma, Chronic Obstructive Pulmonary Disease (COPD), malignancies, Sexually Transmitted Diseases (STDs), cardiac disease, renal diseases, hepatic diseases, myocardial infarction, gout and arthritis were excluded from the study.
Collection of sample: The individuals were requested for overnight fasting. Seven mL of blood samples were drawn with a disposable syringe and collected in a clean, disposable plastic tube from an anterior cubital vein. The serum was separated and lipid profile was done with the fresh sample and rest of the sample was stored at -20°C till analysis.
Parameters Measured
Anthropometric measurement: 1. Anthropometric indices include height and weight. All the individuals were measured wearing light clothing without shoes and hats. Height was measured to the nearest 0.1 cm using a portable stadiometer and weight was measured to the nearest 0.1kg using calibrated platform scales. Body Mass Index (BMI) of the subjects was calculated using standard formula, BMI=Weight (Kg)/{Height (m)}2.
2. Blood pressure measurements: Blood pressure was measured with mercury sphygmomanometer using standard recommended procedures.
Biochemical measurement: 1. Paraoxonase 1 activity was measured by the chemical method, by using phenylacetate as substrate [12].
2. Superoxide dismutase activity was measured by the method of Marklund and Marklund [13].
3. Assay of lipid peroxidation (MDA) by Kei Satoh method [14].
4. Vitamin E was estimated by the method of Martinek GR [15] and Vitamin C by the method of Burtis CA [16].
5. Lipid parameters were estimated by the enzymatic method by fully autoanalyser (CPC Turbochem 100).
Results
The mean values of anthropometric parameters of control and hypertensive subjects are depicted in [Table/Fig-1]. [Table/Fig-1] shows that the BMI, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and WHR (waist-hip circumference ratio) increased significantly in hypertensive subjects as compared to controls. The mean values of lipid parameters (CHO, TG, LDL, and VLDL) PON 1, Vitamin E and C, SOD and MDA are depicted in [Table/Fig-2]. The mean levels of all the lipid parameters except HDL and MDA increased significantly whereas HDL, SOD, PON 1 and Vitamin E and C decreased significantly in hypertensive patients as compared to controls.
Changes in anthropometric parameters in hypertensive subjects.
| S. No. | Variants | Hypertensive patients | Healthy Controls | p-value |
|---|
| 1 | Age (years) | 43.91±7.82 | 42.48±4.81 | 0.12 |
| 2 | BMI (kg/m2) | 27.70±3.19 | 24.92±3.29 | <0.001* |
| 3 | SBP (mmHg) | 160.15±6.47 | 113.86±4.99 | <0.001* |
| 4 | DBP (mmHg) | 100.15±3.66 | 81.42±3.82 | <0.001* |
| 5 | WHR | 0.989±0.049 | 0.80±0.057 | <0.001* |
*p<0.05 considered as statistically significant
Changes in biochemical parameters in hypertensive subjects.
| S. No. | Variants | Hypertensive patients | Healthy Control | p-value |
|---|
| 1 | Total Cholesterol (mg/dL) | 264.30±14.40 | 197.75±23.21 | <0.001* |
| 2 | Triglyceride (mg/dL) | 221.28±35.59 | 125.93±18.72 | <0.001* |
| 3 | VLDL-c (mg/dL) | 44.26±7.12 | 25.19±3.74 | <0.001* |
| 4 | HDL-c (mg/dL) | 33.70±5.42 | 48.82±7.68 | <0.001* |
| 5 | LDL-c (mg/dL) | 186.35±15.78 | 123.75±21.18 | <0.001* |
| 6 | MDA (nmol/mL) | 3.73±0.68 | 2.92±0.46 | <0.001* |
| 7 | Vitamin E (mg/dL) | 1.41±0.11 | 1.76±0.25 | <0.001* |
| 8 | Vitamin C (mg/dL) | 1.33±0.133 | 1.57±0.18 | <0.001* |
| 9 | PON 1 (U/mL) | 50.95±9.67 | 67.61±7.45 | <0.001* |
| 10 | SOD (U/ml) | 8.74±1.29 | 10.88±1.87 | <0.001* |
*p<0.05 considered as statistically significant
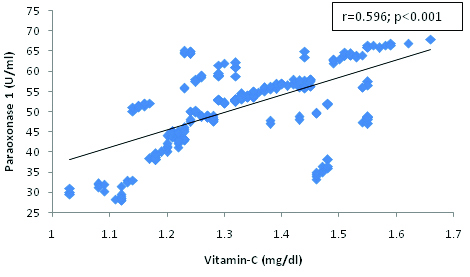
[Table/Fig-3,4,5,6 and 7] shows correlation analysis between the studied subjects. Paraoxonase 1 was significantly and positively correlated with HDL-c, SOD, vitamin E and C whereas, it was significantly and negatively correlated with MDA.
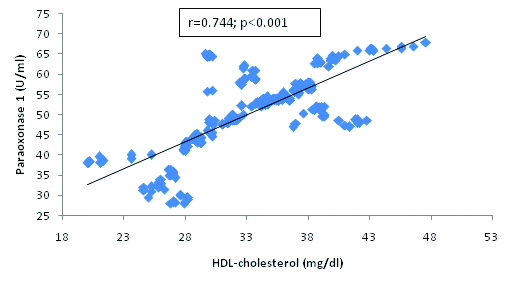
Correlation between Paraoxonase 1 and HDL-cholesterol in hypertensive subjects.
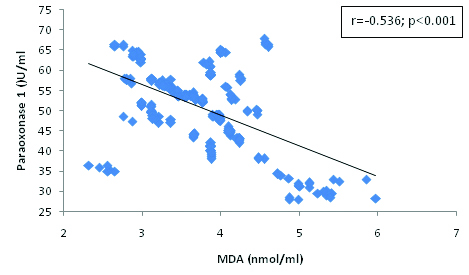
Correlation between Paraoxonase 1 and MDA in hypertensive subjects.
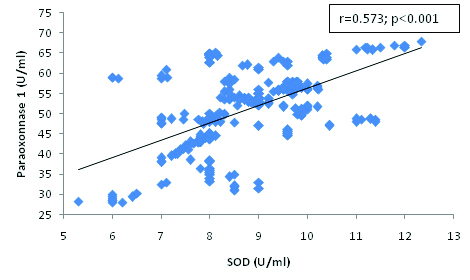
Correlation between Paraoxonase 1 and SOD in hypertensive subjects.
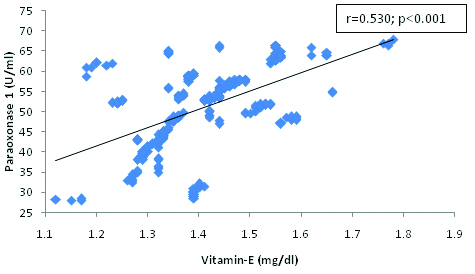
Correlation between Paraoxonase 1 and Vitamin E in hypertensive subjects.
Correlation between Paraoxonase 1 and Vitamin C in Hypertensive subjects.
Discussion
The present study showed that BMI and WHR were significantly higher in hypertensive subjects as compared to the controls. The hypertensive group had significantly higher fasting cholesterol LDL-c, TG, glucose, and blood pressure levels and decreased levels of HDL-cholesterol as compared to controls. Insulin plays a central role in determining triglyceride clearance from the blood via activation of lipoprotein lipase and triglyceride output through effects on the synthesis and secretion of VLDL by the liver. It is thought that in the insulin-resistant state, triglyceride-rich lipoproteins accumulate in the circulation due to decreased activity of lipoprotein lipase, increased lipolysis in adipose tissue, and increased output of VLDL particles from the liver [17,18]. Our results are in accordance with Saxena T et al., [19] and Choudhury KN et al,. [20].
We found a significantly higher level of MDA and significantly decreased levels of SOD, Vitamin E and C, PON 1 in hypertensive patients as compared to healthy controls. The increased oxidative stress levels that we observed in hypertensive patients are consistent with the findings of previous studies [21-23]. The occurrence of oxidative stress may arise from a primary decrease in the antioxidant defence system activity or from an elevation of ROS concentration. This derangement causes oxidative damage to the structure of biomolecules, which involve the antioxidant enzymes, thus, contributing to the oxidative stress found in hypertensives, but not in healthy individuals. As a consequence of increased ROS, a reduction in the endothelium-dependent vasodilation of the vascular smooth muscle cells of hypertensives could be expected [24,25]. In turn, elevation in blood pressure could also contribute to the increase of ROS, thereby enhancing the mechanism of ROS-mediated hypertension through a complex interdependency.
It was observed by Nakazono K et al., that administration of SOD in hypertensive rats lead to a decrease in the blood pressure while that its administration in normotensive rats does not bring any change in the blood pressure levels suggesting that the SOD eliminates the ROS and relative increase in ROS produces hypertension [26].
Kumar A et al., observed that the activity of PON1 decreases and oxidative stress markers namely MDA and conjugated diene increase in hypertensive subjects [27]. Jena D et al., found the PON 1 activity to be directly proportional to total antioxidant capacity and correlated negatively with total antioxidant load in hypertensive subjects [28].
In the atherosclerotic vascular disease, there is an important role played in the initiation and progression by the increased oxidative stress. Oxidative modification of LDL is inhibited by the PON 1, which is an antioxidant enzyme, and has a contribution in most of the antioxidant activity of HDL. In our observation, PON activity was found to be significantly lowered in the hypertensive subjects than in controls and there is a significant negative correlation between PON1 levels and BMI as well as with MDA level and positive correlations between PON 1 and HDL-cholesterol, antioxidants, namely vitamin E, Vitamin C and SOD.
The present findings demonstrate a correlation between oxidative stress markers and paraoxonase 1 activity. We found a significant and negative correlation between PON 1 and MDA [Table/Fig-4] and the significant positive correlation between PON 1 and HDL-cholesterol [Table/Fig-3], SOD [Table/Fig-5], Vitamin E [Table/Fig-6] and C [Table/Fig-7]. The correlation of PON 1 with oxidative stress-related parameters in hypertensive subjects suggested that these parameters have an additional blood pressure–modulating effect. Essential hypertensive subjects showed an impairment of the antioxidant defence system as assessed by a diminution of plasma antioxidant status and increased oxidants level, is in agreement with previous data [29,30].
Limitation
The usefulness of the results of the present study may be limited because we have used only male subjects of 30-60 years age. It is well-known that endogenous and exogenous oestrogens and progestogens, which could be subjected to time-dependent variations in women, are able to modulate renal sodium metabolism as well as the activity of the renin-angiotensin-aldosterone axis [31]. However, future studies are needed in order to get more precise results.
Conclusion
Our study concludes that PON 1 activity is decreased and oxidative stress is increased in hypertension. We found a negative correlation between MDA and PON 1 and a positive correlation between PON 1 and HDL-cholesterol, SOD, vitamin E and C. Diet rich in antioxidant, antioxidant therapy and antilipid drugs may also help to raise PON 1 level, thereby reducing morbidity and mortality in hypertensive patients.
*p<0.05 considered as statistically significant
*p<0.05 considered as statistically significant