Dengue virus infection is an infectious disease transmitted by female mosquitoes, especially Aedes aegypti and A. albopictus species, and has been spreading worldwide. World Health Organisation (WHO) reported that dengue incidence has increased dramatically in the last 50 years and continue to spread to more than 125 countries and a new projection using cartographic approaches propose this number is almost 400 million around the globe [1].
Dengue disease is caused by DENV infection, which is divided into four serotypes namely DENV1, DENV2, DENV3, and DENV4, respectively. To reduce the fatality cases caused by the infection, an appropriate management such as an early and effective diagnosis is required [2]. Several studies describe the attempts to utilise E, NS1 and NS3 protein as the target for preventive, therapeutic, and diagnostic approaches [3-6]. Among these proteins, the soluble form of NS1 protein commonly used as the target for early diagnosis of DENV infection. This protein circulates in the bloodstream from day one after the onset of symptoms until day nine [7].
The sensitivity and specificity of several commercial NS1 assay in detection of confirmed dengue infection sera have been reported between 76.76-98.8% and 98.31-100%, respectively [8-10]. In general, sensitivity and specificity of NS1 detection assay demonstrate their potential role as a cost-effective and acceptable alternative method to another assay such as RT-PCR for the diagnosis of dengue infection in a primary healthcare setting [8].
The detection of NS1 antigen from patient serum requires monoclonal antibodies anti-NS1 which are conjugated with colloidal gold on the strip of lateral flow assay platform or coated on ELISA plate. In this study, we reported the performance of monoclonal antibody anti-DENV NS1 generated from the mice which immunized directly with the lysate of mammalian cells expressing DENV3 NS1 protein. The production of anti-NS1 monoclonal antibodies by using DENV3 NS1 proteinin this study is expected to be used in detecting native NS1 antigen of all DENV serotypes.
Materials and Methods
Animal experiments were carried out in animal facilities of Center for Pharmaceutical and Medical Technology, Agency for the Assessment and Application of Technology (BPPT) from May to July 2017. The study protocol was approved by the Health Research Ethics Committee of Faculty of Medicine, University of Indonesia (364/UN.F1/ETIK/2017).
Cells and Viruses
Myeloma PAI cells were maintained in RPMI 1640 (Gibco) medium supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin-streptomycin and incubated at 37°C with 5% CO2. CHO-K1 cells were cultured in Minimum Essential Medium Eagle with alpha modifications (MEM-α) (Gibco) containing 10% FBS, and 1% penicillin-streptomycin. DENV strains from Indonesian clinical isolates were used in this study; DENV1 RDS 59/09, DENV2 R&D 017, DENV3 IDS 39/10, and DENV4 ITD 08. All DENV were propagated in Vero cells grown in MEM (Gibco) medium with 2% FBS and 1% Penicillin-streptomycin at 37°C and 5% of CO2.
NS1 Protein Preparation
NS1 recombinant protein from a DENV-3 Indonesian clinical isolate was cloned into pFLAG-CMV4 in previous study (due for publication). As much as 20 μg of pFLAG-CMV-NS1 was transfected into 3×106 cells CHO-K1 cells in T-75 Flask and incubated at 37°C with 5% CO2 for two days. Cells were lysed in 200 μL of phosphate saline buffer pH 7.4 and used for mice immunization (one dose for one mice).
Hybridoma Preparation
Mouse monoclonal antibody was prepared by following the procedure of hybridoma preparation [11]. Balb/c mice (4-6 weeks) was immunized with 200 μL of lysed expressing NS1 via intraperitoneal route. Boosters were given three times every two weeks with the same dose. Serum was obtained three days after each boosting and the presence of anti-NS1 polyclonal antibodies were detected by ELISA. Splenocytes were collected from immunized mice after the last boosting and the fusion was performed with mouse myeloma PAI cells. The fused cells were grown on selective DMEM-HAT medium and the hybridomas were screened by indirect ELISA. The limiting dilution was carried-out for the hybridomas which showed positive results in the first screening.
Indirect ELISA
The detection of polyclonal antibody anti-NS1 from the mice serum and the first screening to determine the hybridoma secreting anti-NS1 antibody was carried out by indirect ELISA [12]. A 96-well plate was coated with 100 μL of recombinant NS1 protein (purified with a His-Trap affinity chromatography column, concentration of 1 μg/mL in PBS) for overnight at 4oC. The plate was blocked with PBS-Tween-20 0.1% (PBS-T) containing 5% BSA and incubated at 37°C for 3 hours. The plates were washed with PBS-T and incubated with mice serum or hybridomas supernatant and polyclonal antibody to dengue NS1 (GTX103346 GeneTex) as positive control, respectively. HRP-conjugated anti-mouse IgG was used as secondary antibody (Abcam). TMB substrate was added and Optical Density (OD) was measured at 450 nm.
Immunofluorescence Assay (IFA) of Anti-NS1 mAbs
CHO-K1 cells were seeded in 96-well plate and the next day the cells were transfected with 0.5 μg/well of pFLAG-CMV4-NS1 and incubated at 37oC for two days in the presence of 5% CO2. Supernatant of hybridoma was used as primary antibody and goat anti-mouse IgG-Alexa Fluor 488 (ThermoScientific) as the secondary antibody. The plates were observed under fluorescence microscope and green fluorescent cells indicates the interaction of mAbs with NS1 protein.
Western Blot Analysis of Anti-NS1 mAbs
The western blot protocol was carried-out by slightly modified from previous report [13]. DENV serotype 1-4 were propagated in Vero cells. Five days post infection, the infected Vero cells were lysed, loaded into 12% acrylamide gel and transferred to Polyvinylidene Difluoride (PVDF) membrane. (PVDF) membrane was incubated with monoclonal antibody overnight at 4°C, followed by HRP-conjugated anti-mouse IgG antibody. Femto luminol substrate was added and the reactivity of the monoclonal antibody to detect the NS1 antigen was observed on the film.
Determination of the Dissociation Constant
Determination of the dissociation constant (Kd) of antibody-antigen equilibria in solution was carried out by competitive ELISA [14]. In this ELISA procedure, 50 μL/well of antigen at 0.5 μg/mL in Phosphate Buffered Saline with Tween-20 (PBS-T) was coated on ELISA plate. The plate was wrapped with cling film and incubated at 4°C for overnight at 4oC. At the same time, in microcentrifuge tubes, setup 200 μL (for triplicate) of two-fold dilutions of the NS1 antigen (Ab64456, Abcam) in PBS-T with 1% BSA (111.11 nM to 0.86 nM) mixed with 0.3 μg/mL of a pre-determined amount of specific antibody. The antibody/antigen mixture was incubated in rotary shaker with the speed of 4 rpm for 20 hours at 17oC. The plate was blocked with PBS-T containing 5% BSA and incubated at 37°C for 3 hours. The plates were washed with PBS-T and incubated at 37°C for 1 hour with 50 μL/well of the mixture antibody/antigen (in triplicate for each assay point). HRP-conjugated anti-mouse IgG was used as secondary antibody (Abcam). TMB substrate was added and OD was measured at a 450 nm. Analysis of binding data and determining the dissociation constant using GraphPad Prism 5.0 software (La Jolla, California, USA).
Results
Polyclonal Antibodyanti-NS1
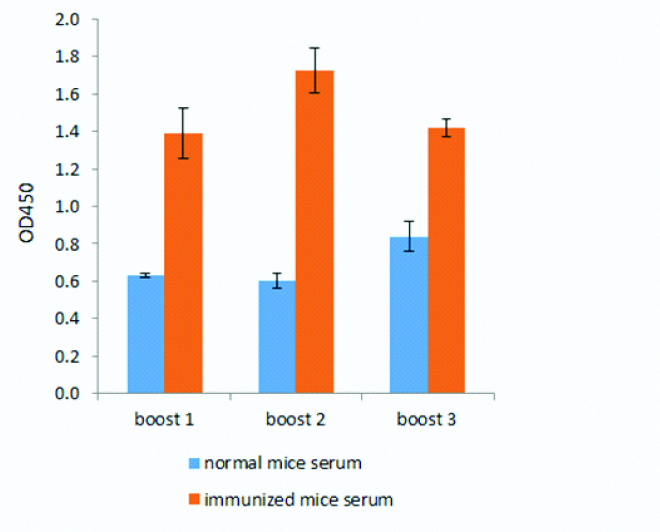
The difference of the mean value of optical density between immunized and non-immunized serum indicated that NS1 protein has successfully induced immune response in mice [Table/Fig-1]. The optical density of normal mice serum were 0.63, 0.60, 0.84 and for the immunized mice serum were 1.39, 1.73, 1.42 for boost 1, 2, and 3, respectively.
Polyclonal antibody (PAb) anti-NS1. Detection of PAb anti-NS1 antibody from immunized mice (red bar) and non-immunized mice (blue bar).
Hybridoma of Anti-NS1 Monoclonal Antibody
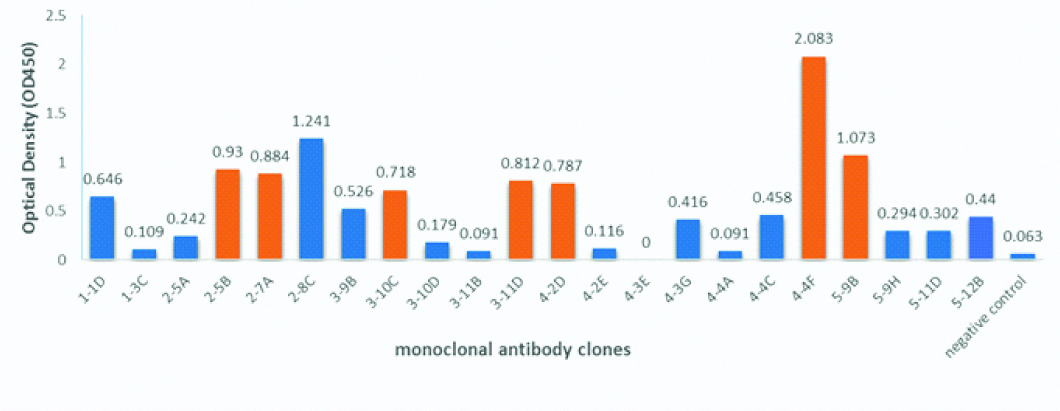
Sixteen monoclonal antibody anti-NS1 clones showed a high ELISA signal with values between 0.303 and 2.08 [Table/Fig-2] against the DENV3 recombinant NS1 protein. Seven out of sixteen clones (2-5B, 2-7A, 3-10C, 3-11D, 4-2D, 4-4F, 5-9B) were selected for further analysis by Immunofluorescence Assay (IFA) and Western blot methods to confirm the reactivity of the antibody. One clone, 2-8C, was excluded for further analysis since the growth of the hybridoma was very slow compare to the other clones.
Screening of potential hybridoma. Sixteen hybridomas were selected and analysed the ability of clones to produce antibody anti-NS1. Seven clones indicated by the red bar were selected for further analysis.
Immunofluorescence Assay (IFA)
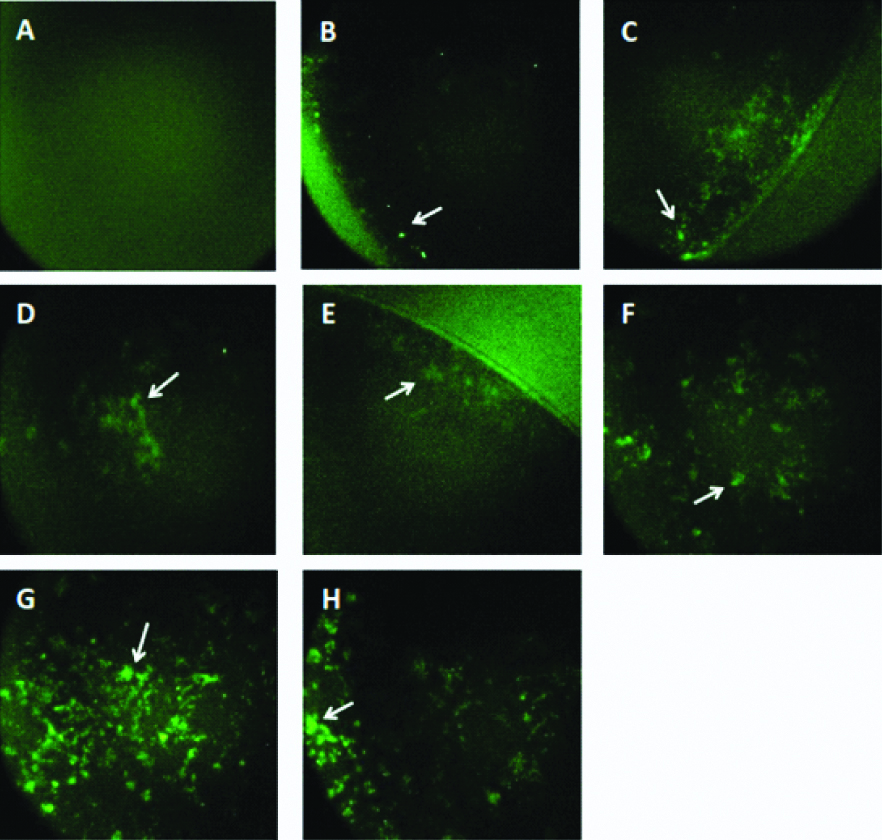
Reactivity of monoclonal antibodies to detect the native form of NS1 protein were tested by using IFA. Six out of seven clones (2-7A, 4-2D, 3-11D, 3-10C, 4-4F, 5-9B) showed positive results as shown in [Table/Fig-3].
Immunofluorescence assay of mAbs; a,b) CHO-K1 (mock cells) and pCMV4-NS1 transfected CHO-K1 were incubated with commercial NS1 (GeneTex, GTX4212) as positive control; c) mAb 2-7A; d) mAb 4-2D; e) mAb 3-11D; f) mAb 3-10C; g) mAb 4-4F; and h) mAb 5-9B. Positive signals were indicated by the green fluorescence signals.
Western Blot
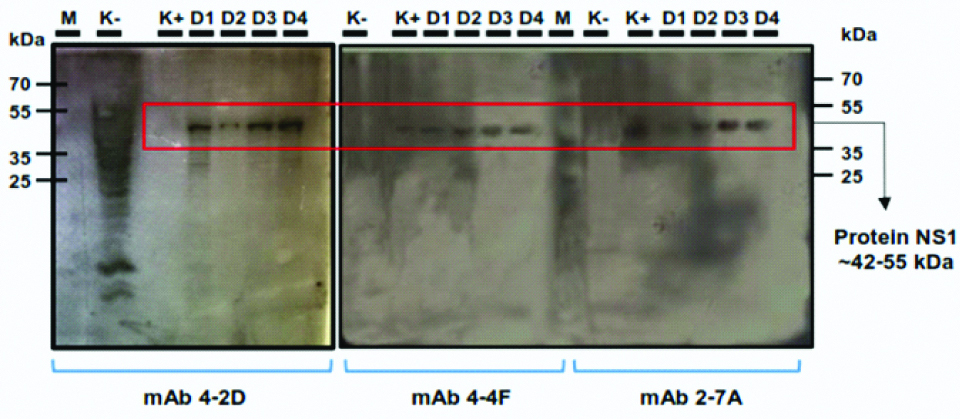
Verification of the monoclonal antibody to detect the NS1 antigen from the DENV were carried-out by using western blot analysis. We tested the reactivity of three clones (4-2D, 4-4F, 2-7A) to detect NS1 antigen from DENV infected Vero cells. All three clones could detect specific NS1 antigen (42-55 KDa) from all DENV serotype as shown in [Table/Fig-4].
Western blot analysis. Non-infected Vero cells lysate (K-), commercial NS1 protein (GeneTex, GTX4212), 0,5 μg/mL (K+) and DENV infected Vero cells (D1-D4) were transferred onto nitrocellulose membranes and incubated with supernatant media of clones 4-2D, 4-4F and 2-7A. Positive results were indicated by the appearance of specific band around 42-55 KDa.
Determination of the Dissociation Constant
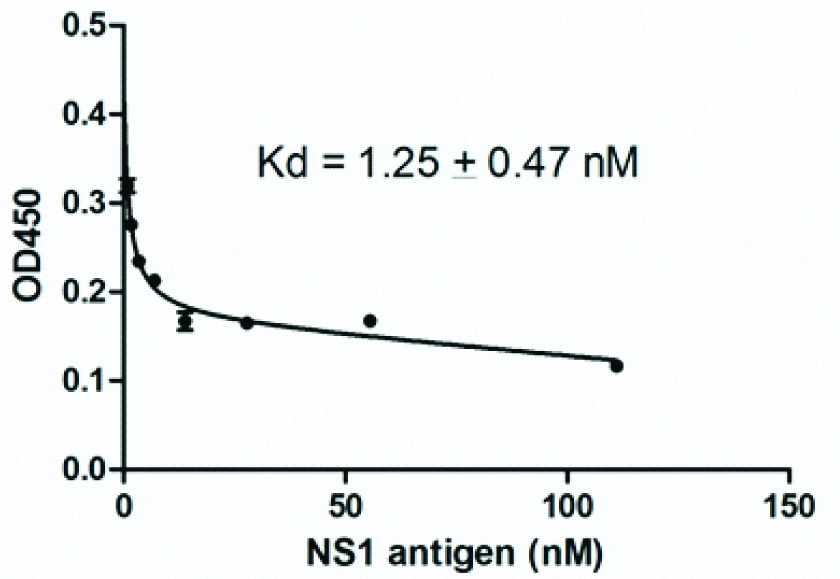
A constant amount of 4-4F monoclonal antibody (0.3 μg/mL) was incubated in solution with various amounts of NS1 antigen until equilibrium was reached. The unsaturated antibody from each antigen concentration was measured by ELISA. Dissociation constant (Kd) of 4-4F clone was calculated by curve fitting by non-linear regression with saturation one-site model using GraphPad Prism 5.0 software. Clone 4-4F clearly recognised the NS1 antigen and showed a strong affinity at 1.25±0.47 Nm [Table/Fig-5].
Binding affinity of 4-4F monoclonal antibody against NS1 antigen. Dissociation constant (Kd) of 4-4F clone was calculated by curve fitting by non-linear regression with saturation one-site model using GraphPad Prism 5 software and showed a strong affinity to NS1 antigen at 1.25±0.47 nM.
Discussion
The use of CHO mammalian cells as protein expression system has several advantages over prokaryotic expression systems and unicellular eukaryotes (yeast cells). CHO cells are adaptable and easily grown cells in a suspension culture medium, often used in large-scale industries. CHO cell is a well-known expression system that widely used in the production of therapeutic proteins. This type of cell has a low risk of being infected with the virus that causes the disease in humans. CHO cells can be efficiently transfected with commercial transfection reagents, and are able to express large quantities of recombinant proteins. CHO cells are also capable of post-translation modification of compatible recombinant and bioactive proteins with humans [15]. Based on this information, we expressed the DENV3 NS1 protein in CHO-K1 cells with expectation the recombinant protein will have a structure and conformation similar to the native NS1 in the body of DENV-infected patients.
A previous study described the use of antigen-expressing mammalian cells as an effective inducer for obtaining monoclonal antibody for malaria detection [16]. In this study, we utilised the NS1 expressing CHO-K1 cells as the inducer for Balb/c mice immunization step and fused the splenocyte with myeloma PAI cells in order to generate the mouse monoclonal antibody anti-DENV NS1 antigen.
After immunization, we evaluated the polyclonal antibody response from immunized and non-immunized mice. The polyclonal antibody response from booster 1 to booster 2 in immunized mice increased compared to the non-immunized as shown in [Table/Fig-1]. However, after booster 3, the antibody response was slightly decreased when compared to booster 2. In a previous study mentioned that immunization with non-adjuvant antigen will induce the peak of antibody response at 2nd to 3rd week after induction and gradually the titer of antibody begins to decrease. While immunization with an adjuvant-antigen is usually reach the peak response after a booster given at least four weeks after the first immunization [17].
In the first screening, we selected 30 hybridomas from the fusion cells and evaluated their ability to detect NS1 protein by ELISA. The positive hybridomas were subjected to limiting dilution and the positive monoclonal clones were evaluated again by ELISA. Around 16 monoclones were positive [Table/Fig-2] and the reactivity of the antibody from the clones to detect the native form of NS1 protein were further examined by IFA. Among the 16 clones, only 6 clones were able to detect the NS1 antigen expressed in CHO-K1 cells [Table/Fig-3]. The intensity of fluorescence for each mAb varies, indicating the level of mAb reactivity with expressed antigens is not equal to each other. MAb 4-4F and 5-9B clones showed a strong signal compared to the others clones. This result was in accordance with the previous screening results by ELISA which showing the highest reactivity to NS1 protein were achieved by 4-4F and 5-9B clones with the reactivity value 2.083 and 1,073, respectively.
In contrast, clone 2-5B showed a high absorbance in the screening step (0.930) by ELISA, however the clone did not show any fluorescence signals by IFA. This may be due to mutations in hybridoma cells or other factors such as changes in antibody conformation due to denaturation, post-translation modification, pH effect, temperature, salt concentration, and fixation processes in IFA procedures during the selection and characterisation steps [18]. Despite intensive cell maintenance and repeated clonal propagation, some cell lines still lose their ability to produce antibodies. It is common for 50-70% of the original cell culture to show positive results to be lost or damaged [19].
We selected three clones (4-2D, 4-4F and 2-7A and carried-out further analysis to determine the ability of the monoclonal antibody to detect NS1 antigen from DENV3 and other DENV serotypes. Cross-reactivity analysis was carried-out using Vero cells, which infected with all four DENV serotypes. The antibodies were reacted to all serotypes of infected cells by western blot analysis [Table/Fig-4]. The result from western blot analysis showed the appearance of specific band corresponds to predicted NS1 antigen at 42-55 kDa from the lysate of all DENV infected cells. The cross-reactivity of the monoclonal antibody to detect endogenous NS1 antigen of four DENV serotypes probably due to the high homology level at amino acid sequences level among all serotypes is around 70%. A previous report shows that induced B-cells from the immunized mice with mammalian expressing NS1 antigen of one stereotype could produce humoral immune response that specific serotypes and also cross-reacted to other serotypes [20]. Furthermore, we performed competitive ELISA to evaluate the binding affinity of monoclonal antibody against the NS1 antigen. One clone, 4-4F, showed a low Kd value around 1.25±0.47 nM [Table/Fig-5]. This result probably indicated that the clone has a strong affinity against NS1 antigen and could be promising to be used in diagnosis application.
Limitation
The information about the properties and characterisation of 4-4F antibody still is limited due to the stability of 4-4F clones during the maintenance and upscale production. Further studies are required, such as epitopes mapping and also pairing assay, in an attempt to develop sensitive diagnostic test using the lateral flow immunochromatography platform.
Conclusion
Monoclonal antibodies which obtained in this study could recognise the NS1 recombinant protein and also native NS1 antigen from all DENV serotypes. The low Kd value of clone 4-4F (1.25±0.47 nM) could be an indicator that monoclonal antibody has a strong binding affinity to NS1 antigen. These findings make our antibodies as a potential biological material that could be used in the development of a dengue detection system.