The prevalence of antimicrobial resistance differs from one region to another and increases with time in many areas. Besides that, decline in eradication rate is reported all over the world [9].
This study was conducted with an aim to determine demographic, clinical and endoscopic presentation of Egyptian adults infected with H. pylori as well as characteristics of patients resistant to eradication therapy commonly used in Egypt. Therapy used is concomitant non bismuth quadruple (PAMC) which include PPI (BID) + amoxicillin (1000 mg BID)+metronidazole (500 mg BID)+clarithromycin (500 mg BID) for 14 days or triple therapy which include PPI (BID)+amoxicillin (1000 mg BID)+clarithromycin (500 mg BID) or PPI (BID)+metronidazole (500 mg BID)+clarithromycin (500 mg BID) for 14 days.
Materials and Methods
A retrospective observational study was conducted at Internal Medicine Department, Endoscopy Unit, Zagazig University Hospital; a tertiary referral centre over a period of six months from June to December 2017 in which 202 patients were enrolled. Institutional ethical clearance was taken. Analysis of data for all adult patients confirmed to have H. pylori-associated chronic active gastritis by gastric biopsy was done [Table/Fig-1].
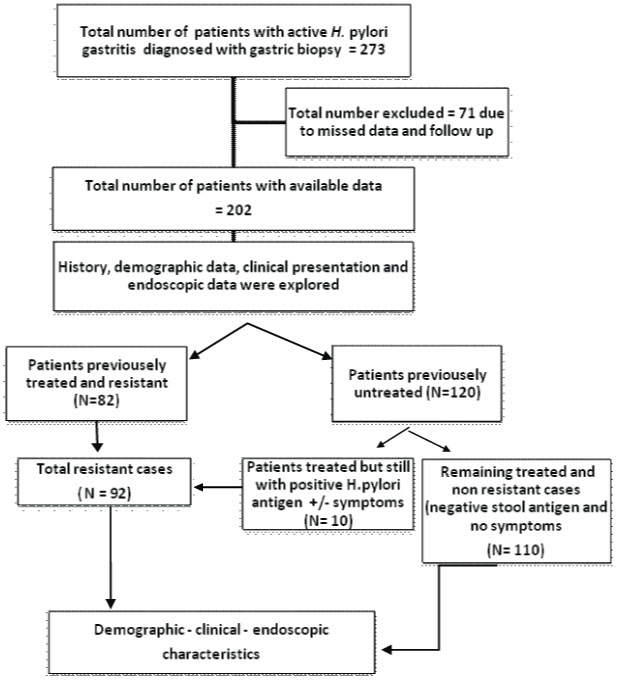
Inclusion criteria: (1) Patients with H. pylori-associated chronic active gastritis, (2) Patients with different age groups and both male and female were included.
Exclusion criteria: (1) Patients with renal failure or cirrhosis with dyspeptic symptoms, (2) Patients with malignancy, (3) Patients with a long history of NSAIDs intake, (4) Patients diagnosed and treated for H. pylori without endoscopic evaluation, (5) Patients who underwent endoscopic examination without a biopsy, (6) Patients with inactive gastritis by biopsy (absence of neutrophils or bacilli infiltration), (7) Unavailability of data and patients who refused to participate were excluded.
The diagnosis of H. pylori infection was done through endoscopic examination with multiple antral, gastric body and fundic biopsies for histopathological examination. Histopathologic analysis was performed with Haematoxylin and Eosin and Giemsa stains. All specimens were assessed for the presence of H. pylori bacilli, mononuclear cells (lymphocytes, plasma cells, and histiocytes) as markers of inflammation and neutrophils as markers of activity, associated metaplasia, atrophy or cancer [10].
Endoscopic Examination
It was done by ordinary white light endoscopy. A CD video was obtained for every patient when applicable for documentation and future re-evaluation. The complete report about the oesophagus, stomach, and duodenum was prepared after examinations by at least 3 endoscopists with an interobserver agreement.
Indications of endoscopic examination: Evaluation of symptoms such as persistent epigastric or heartburn, persistent dyspeptic symptoms as upper abdominal distension or anorexia, patients with alarm symptoms such as bleeding, loss of weight or persistent vomiting and patients with unexplained iron deficiency anaemia.
Patients resistant to treatment were defined as persistence of active inflammation of H. pylori in their biopsy specimens or positive stool antigen in some cases with persistent symptoms after trials of eradication therapy. Drug resistance is the most important cause for treatment failure when H. pylori is not eradicated in a particular patient, after exclusion of lack of adherence [11,12].
Non-resistant cases were defined as an improvement of symptoms and negative H. pylori stool antigen following eradication therapy [13,14].
Symptomatic patients with false negative H. pylori stool antigen either before endoscopic diagnosis and treatment or following eradication therapy in remaining symptomatic patients and biopsy confirmed H. pylori-associated gastritis were also included.
Endoscopic gastritis was defined when the mucosa of the stomach was erythematosus, inflamed or with nodularity [9]. Distribution of inflammation, erosions, ulcers, submucosal vasculature were all examined as well as other associated findings as GERD, malignancy, polyps, and duodenitis were also examined.
Six samples were taken, two from the antrum, two from the body of the stomach and two from fundus for histological examination to avoid patients with patchy H. pylori distribution.
Patient assessment: Patients were subjected to (1) Full history taking, (2) Full clinical examination and collecting data for their clinical presentations, (3) Abdominal ultrasound examination before endoscopy, (4) Collecting data of their upper GIT endoscopic and histological reports, (5) Revision of H. pylori stool antigen results.
Statistical Analysis
The collected data were computerised and statistically analysed using SPSS program (Statistical Package for Social Science) version 24.0. Data were tested for normal distribution using the Shapiro Walk test. Qualitative data were represented as frequencies and relative percentages. Quantitative data were expressed as Mean±SD (Standard Deviation) and median (range). Chi-square test (χ2) and Fisher exact was used to calculate the difference between qualitative variables as indicated. Forward multivariate logistic regression analysis model was done using any predictor with p<0.05 in the univariate analysis. The significance level for all above mentioned statistical tests was done. A p-value ≤0.05 indicates significant, p <0.001 indicates highly significant difference while, p> 0.05 indicates non-significant difference.
Results
Demographic and clinical presentation of enrolled patients: Mean±SD of age was 36.5±14.5 years with a age range of 13-47 years. The study included 138 females (68.3%) and 64 males (31.7%).
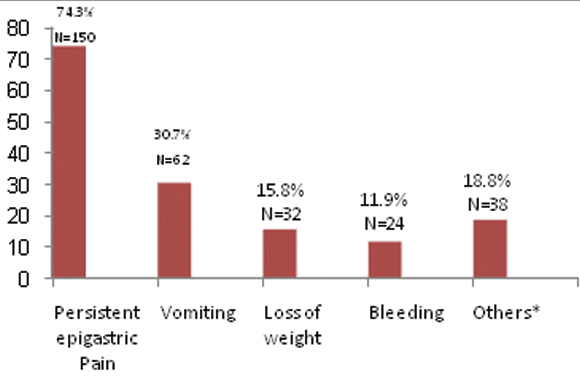
The main clinical presentation for active H.pylori associated gastritis and the main indication for referral to endoscopy unit was the persistent epigastric pain, followed by vomiting, loss of weight and other dyspeptic symptoms or unexplained iron deficiency anaemia. Upper GI bleeding was responsible for 11.9% of clinical presentation and endoscopic examination [Table/Fig-2].
Frequency of clinical presentation and indications for endoscopic examination.
* Other presentations as (persistent eructation, upper abdominal distension, heartburn and unexplained Iron deficiency anaem
Endoscopic findings: For endoscopic gastritis, distribution was mainly pan gastritis and occurred in 76.2% of patients. The main feature was diffuse erythematous mottling and mucosal nodularity was present in 21.8% [Table/Fig-3].
Frequency of Endoscopic findings in studied subjects.
| Endoscopic findings | | No (total=202) | % |
|---|
| Distribution of gastritis | Pan gastritis | 154 | 76.2% |
| Antral | 48 | 23.8% |
| Erythematous mottling | 198 | 98.0% |
| Nodularity | 44 | 21.8% |
| Apparent submucosal vasculature mainly fundic | 80 | 39.6% |
| Associated erosions | 46 | 22.8% |
| Associated ulcer | 46 | 22.8% |
| Cancer | 2 | 1.0% |
| Associated GERD | Absent | 102 | 50.5% |
| C | 8 | 4.0% |
| B | 10 | 5.0% |
| A | 82 | 40.6% |
| Associated duodenitis | Severe | 42 | 20.8% |
| Moderate | 10 | 5.0% |
| Mild | 106 | 52.5% |
| Absent | 44 | 21.8% |
| Incompetent cardia | 180 | 89.1% |
| Associated polyps | 12 | 5.9% |
Qualitative data were represented as frequencies and relative percentages
Associated features were the presence of apparent submucosal vasculature in 39.6%, erosions and ulcers in 22.8% for both lesions, hyperplastic polyps in 5.9%. Prevalence of cases complicated with adenocarcinoma was only 1%. Incompetent cardia was present in 89.1% while GERD was present in 49.5% mainly of Type A. Duodenitis occurred in 79.2% mainly of a mild type [Table/Fig-3].
Histological findings: Mild activity was detected in 98% of patients while moderate activity was present in 2%. Atrophic changes were found in only 2% and malignancy in 1% of cases. No cases with dysplasia or metaplasia were found.
Comparison between males and females according to clinical presentations and endoscopic findings: Vomiting and weight loss were significantly higher in females compared to males (p-values 0.012 and 0.007, respectively), while there were no significant differences between them as regard to other presentations (p>0.05) [Table/Fig-4].
Comparison between males and females according to clinical presentations and endoscopic findings.
| Clinical presentation | Female (138) | Male (64) | p-value |
|---|
| n | % | n | % |
|---|
| Vomiting | 50 | 36.2% | 12 | 18.8% | 0.012 |
| Persistent epigastric pain | 102 | 73.9% | 48 | 75.0% | 0.869 |
| Loss of weight | 28 | 20.3% | 4 | 6.3% | 0.007 |
| Bleeding | 14 | 10.1% | 10 | 15.6% | 0.263 |
| Others | 24 | 17.4% | 14 | 21.9% | 0.448 |
| Endoscopic features |
| Distribution of gastritis | Pan gastritis | 112 | 81.2% | 42 | 65.6% | 0.016 |
| Antral | 26 | 18.8% | 22 | 34.4% |
| Erythematous mottling | 136 | 98.6% | 62 | 96.9% | 0.426 |
| Nodularity | 38 | 27.5% | 6 | 9.4% | 0.004 |
| Apparent submucosal vasculature mainly fundic | 56 | 40.6% | 24 | 37.5% | 0.677 |
| Associated erosions | 30 | 21.7% | 16 | 25.0% | 0.607 |
| Associated ulcer | 26 | 18.8% | 20 | 31.3% | 0.05 |
| Cancer | 1 | 0.007% | 1 | 0.015% | 0.06 |
| Associated GERD | No | 70 | 50.7% | 32 | 50.0% | 0.618 |
| C | 4 | 2.9% | 4 | 6.3% |
| B | 6 | 4.3% | 4 | 6.3% |
| A | 58 | 42.0% | 24 | 37.5% |
| Associated duodenitis | Severe | 30 | 21.7% | 12 | 18.8% | 0.916 |
| Moderate | 6 | 4.3% | 4 | 6.3% |
| Mild | 72 | 52.2% | 34 | 53.1% |
| No | 30 | 21.7% | 14 | 21.9% |
| Incompetent cardia | 120 | 87.0% | 60 | 93.8% | 0.149 |
| Associated polyps | 10 | 7.2% | 2 | 3.1% | 0.249 |
Qualitative data were represented as frequencies and relative percentages
Chi-square test (χ2) and Fisher exact was used to calculate the difference between qualitative variables
Endoscopic findings showed that females had significant pan gastric affection as well as mucosal nodularity compared to males (p-values 0.01 and 0.004, respectively) but there was no difference between them as regard to other endoscopic findings (p>0.05) [Table/fig-4].
Comparison between resistant and non-resistant cases to previous eradication therapy according to clinical and endoscopic features: Epigastric pain was significantly higher in patients with resistant H. pylori infection (p-<0.001), while other dyspeptic symptoms were significantly higher in non resistant cases (p=0.001). No significant differences between the two groups as regards other clinical presentations was found (p>0.05) [Table/Fig-5].
Comparison between resistant and non-resistant cases to previous eradication therapy according to clinical and endoscopic features.
| Clinical presentation | Resistance to eradication | p-value |
|---|
| No (N=110) 54.5% | Yes (N=92) 45.5% |
|---|
| No | % | No | % |
|---|
| Vomiting | 28 | 25.5% | 34 | 37.0% | 0.078 |
| Persistent epigastric pain | 66 | 60.0% | 84 | 91.3% | <0.001 |
| Loss of weight | 14 | 12.7% | 18 | 19.6% | 0.185 |
| Bleeding | 14 | 12.7% | 10 | 10.8% | 0.08 |
| Others | 30 | 27.3% | 8 | 8.7% | 0.001 |
| Sex |
| Female | 76 | 69.09% | 62 | 67.3% | 0.7 |
| Male | 34 | 30.9% | 30 | 32.7% |
| Age |
| <40 year | 76 | 69.09% | 62 | 67.3% | 0.41 |
| > 40 year | 34 | 30.9% | 30 | 32.7% |
| Endoscopic finding |
| Distribution of gastritis | Pan gastritis | 76 | 69.1% | 78 | 84.8% | 0.009 |
| Antral | 34 | 30.9% | 14 | 15.2% |
| Nodularity | 22 | 20.0% | 22 | 23.9% | 0.502 |
| Apparent submucosal vasculature | 28 | 25.5% | 52 | 56.5% | <0.001 |
| Associated erosions | 28 | 25.5% | 18 | 19.6% | 0.32 |
| Associated ulcer | 26 | 23.6% | 20 | 21.7% | 0.749 |
| Associated GERD | No | 64 | 58.2% | 38 | 41.3% | <0.001 |
| C | 8 | 7.3% | 0 | 0.0% |
| A | 8 | 7.3% | 2 | 2.2% |
| B | 30 | 27.3% | 52 | 56.5% |
| Associated duodenitis | Severe | 18 | 16.4% | 24 | 26.1% | <0.001 |
| No | 38 | 34.5% | 6 | 6.5% |
| Moderate | 6 | 5.5% | 4 | 4.3% |
| Mild | 48 | 43.6% | 58 | 63.0% |
| Incompetent cardia | 92 | 83.6% | 88 | 95.7% | 0.006 |
| Associated polyps | 4 | 3.6% | 8 | 8.7% | 0.13 |
Qualitative data were represented as frequencies and relative percentages
Chi-square test (χ2) and Fisher exact was used to calculate the difference between qualitative variables
At endoscopy, resistant cases showed significant pan gastric affection than antral with apparent submucosal vasculature (p-values 0.009 and <0.001) [Table/Fig-5].
Resistant cases also show significant associated features as incompetent cardia, GERD, and duodenitis (p-values 0.006, <0.001, <0.001). There was no significant difference between two groups compared to other parameters (p-value>0.05) [Table/Fig-5].
On multivariate logistic regression, we found that epigastric pain, the presence of apparent submucosal vasculature and GERD were independently associated with resistant cases (p values <0.001, 0.001, 0.001 respectively) [Table/Fig-6].
Multivariate logistic regression of potential clinical and endoscopic predictors of resistant cases.
| Predictors | B | SE | p-value | OR | 95% CI for OR |
|---|
| Lower | Upper |
|---|
| Persistent epigastric pain | 2.4 | 0.5 | <0.001 | 11.0 | 4.062 | 29.530 |
| Associated GERD | 1.3 | 0.4 | 0.001 | 3.6 | 1.629 | 7.754 |
| Apparent submucosal vasculature | 1.3 | 0.4 | 0.001 | 3.5 | 1.628 | 7.633 |
| Constant | -3.8 |
β: regression coefficient; SE: standard error; OR: odds ratio; 95%CI: 95% confidence interval; p<0.05 is significant;
Forward multivariate logistic regression analysis model was done using any predictor with p<0.05 in the univariate analysis.
Endoscopic findings in patients with false negative H. pylori stool antigen testing: Pan gastritis was present in 65.2% of patients with diffuse erythematous mottling, while mucosal nodularity was present in 13%. Associated features were the presence of apparent submucosal vasculature in 39.1%, erosions and ulcers in 8.7%, and 21.7%, respectively. Incompetent cardia was present in 95.7% while GERD was present in 53.2% mainly of Type A. Duodenitis occurred in 79.3% mainly of a mild type [Table/Fig-7].
Endoscopic findings in patients with false negative H. pylori stool antigen testing.
| Endoscopic findings | N=46 (22.8%) |
|---|
| No. | % |
|---|
| Distribution of gastritis | Antral | 16 | 34.8% |
| Pan gastritis | 30 | 65.2% |
| Erythematous mottling | 46 | 100.0% |
| Nodularity | 6 | 13.0% |
| Apparent submucosal vasculature | 18 | 39.1% |
| Associated ulcer | 10 | 21.7% |
| Cancer | 0 | 0.0% |
| Associated erosions | 4 | 8.7% |
| Associated GERD | A | 18 | 39.1% |
| B | 2 | 4.3% |
| C | 4 | 8.7% |
| No | 22 | 47.8% |
| Associated duodenitis | No | 10 | 21.7% |
| Mild | 22 | 47.8% |
| Moderate | 4 | 8.7% |
| Severe | 10 | 21.7% |
| Incompetent cardia | 44 | 95.7% |
| Associated polyps | 0 | 0.0% |
Qualitative data were represented as frequencies and relative percentages
Discussion
The World Gastroenterology Organization (WGO) reported that the H. pylori prevalence in Egypt was 90% in adults. In the Middle East, H. pylori prevalence ranges from 60-90% as documented by WGO [4].
In this study, we found that percentage of symptomatic females infected with H. pylori and referred for endoscopic examination was higher than males (68.3%) versus (31.7%). Most H. pylori-related diseases are known to be associated with male, which was not in agreement with present finding. The role of male predominance as a risk factor for H. pylori infection in adults was reviewed by De Martel C and Parsonnet J in a meta-analysis of large, population-based studies [15].
A significant elevation of urea breath test values in infected females was reported compared to males in a recent study [16]. Similar results were reported by Moshkowitz M et al., [17], who found that the number of total referrals for urea breath testing was significantly higher among females than males with significantly higher mean values among them. This may represent an increased bacterial load among females, that causes the decreased response to antibiotic therapy [17]. Moayyedi P et al., [18] reported same results. This higher bacterial load among females may explain why more females in this study were complaining and referred more to endoscopy even following eradication therapy and supported present results. Also significantly increased seropositivity in women (60.6%) compared with men (42.9%) was found in Kanbay M et al., study [19].
The Mean±SD for age in the present study was 36.5±14.5 years closely similar results obtained by Moshkowitz M et al., [17] who found females commonly being referred for dyspeptic symptoms between third and fifth decades of life and also in Emara MH et al., study [20]. The affection of younger age reflects the low socioeconomic state that predisposes to infection.
The main clinical presentation for active H. pylori associated gastritis in present study was the persistent epigastric pain, followed by vomiting, dyspeptic symptoms and loss of weight. Similar results obtained by Rajeswari P et al., who reported that abdominal pain with dyspepsia more than three months is the most common clinical presentation followed by abdominal discomfort with vomiting and nausea and also in agreement with other study [21,22].
Several studies have explored the relationship between symptoms and H. pylori infection. It had been reported that H. pylori (+) patients have higher ulcer-like dyspepsia scores and lower dysmotility-like dyspepsia scores than H. pylori(-) patients [23] and there is a higher prevalence of H. pylori infection in ulcer-like dyspepsia than in dysmotility-like dyspepsia [24]. This explains the lower prevalence of dysmotility-like symptoms as persistent eructation, upper abdominal distension, and heartburn in present study.
Bleeding accounted for 11.9% of presentation; this lower percentage may be explained by widespread use of PPI for a variety of abdominal symptoms and its availability as over counter medication making the low prevalence of bleeding ulcers, near results reported by Rajeswari P et al., [22].
For endoscopic gastritis, distribution was mainly pan gastritis. Previous study done by Emara MH et al., [20], which recruited Egyptian population, showed that gastritis at the time of endoscopy was pan gastritis in 88%, which was similar to current study. Higher prevalence of pan gastric type may reflect the severity of infection.
The higher percentage of mucosal nodularity in this study compared to Emara MH et al., [20] study which was 21.8% versus 13.9% may be explained by the smaller number of active H. pylori gastritis patients included in their study compared to the number of our patients.
The presence of diffuse redness strongly suggests mucosa with current H. pylori infection [25]. Some endoscopic findings such as spotty redness, nodularity, and hyperplastic polyps are known to be associated with H. pylori infection.
Nodular gastritis has been shown to be strongly associated with pangastritis [26]; this was in agreement with the finding of present study.
Prevalence of combined endoscopic erosions and ulcers in this study was 45.6% which was higher than Egyptian patients in Emara MH et al., study [20]. The variations between the two results could be explained in term of the inclusion of a smaller number of patients with positive and active H. pylori in their study. Similar to present study results were present in Rajeswari P et al., [22] study.
A surprising finding here is the presence of apparent submucosal vasculature mainly in the fundus in 39.6%. Endoscopic mucosal atrophy secondary to H. pylori gastritis is diagnosed by visibility of the vascular pattern and rugal atrophy [27]. Despite that very few patients had the diagnosis of mucosal atrophy in histopathological examination, this raises the concept of absence of a good correlation between endoscopic and histological findings and this was in agreement with Al-Hamdani et al., who found that provisional diagnosis of gastric atrophy was proposed in their patients and was pangastric, though the findings suggest gastric atrophy, histopathological findings were consistent with chronic active gastritis and this might be related to the low sensitivity of the white light endoscopic findings or to other additional factors that induce such gross changes [28,29]. Emara MH et al., [20] in the same locality found the same conclusion. Recent advances in endoscopy have resulted in improvement in the diagnosis without histological assessment of biopsied specimens using Image-Enhanced Endoscopy (IEE) [30].
Associated incompetent cardia was present in 89.1% while GERD was present in 49.5% mainly of Type A, GERD was a common association found in Emara MH et al., study also [20], among the group with positive H. pylori stool antigen. Furthermore, similar results were found in Shareef SJ study [31], who found reflux esophagitis in 58.7% and lax sphincter in 77.2% of H. pylori positive cases.
The role of H. pylori infection in GERD is still controversial. This may be due to the fact that H. pylori infection leads to increase acid production which leads to increased oesophageal acid exposure. However, this may be an acceptable explanation in antral predominant gastritis with resultant hypergastrinaemia. In the current study, the gastritis was mainly pan gastric with resultant hypochlorhydria. An explanation for this may be that GERD was prevalent due to obesity, which is commonly found in females or due to prevalent bad lifestyle factors associated with GERD. Another explanation found in the study of WU JC et al., [32], who found that H. pylori-infected patients have more severe oesophageal dysmotility and lower oesophageal sphincter dysfunction, which leads to the development of GERD in these patients and explains present study findings of incompetent cardia in the majority of patients.
Endoscopic duodenitis was a common association with H. pylori in current study which is considered a common finding as H. pylori cause active chronic gastritis and active duodenitis [33].
Vomiting and weight loss were significantly higher in females than males in present study population and this again can be explained by a higher bacterial load in females [17], which cause more aggressive symptoms.
Pan gastric mucosal inflammation and nodularity were also significantly higher in females. Nodularity is common among young female patients [34,35], strongly related to Helicobacter pylori infection and the associated with pan gastritis [26,36]. When endoscopy for a young female shows nodular gastritis, this carries a risk for H. pylori-associated gastric carcinoma; particularly diffuse type [37].
Toronto consensus for the treatment of Helicobacter pylori infection in adults [38], recommended Bismuth quadruple (PBMT) therapy as first-line option for eradication therapy followed by, concomitant non bismuth quadruple (PAMC) or triple therapy which should be restricted to areas with <15% clarithromycin resistance.
In Egypt however, due to unavailability of Bismuth quadruple (PBMT) therapy, the other two options are commonly used as prescribed by the majority of clinicians and even the triple therapy preparation in a single package is available as over counter medication.
In this study, a high resistant rate of 45.5% to these available lines of treatment was found in patients referred for endoscopy and this can be explained with high prevalence of genetic mutations that may confer resistance to metronidazole and clarithromycin secondary to the misuse of these antibiotics in Egypt [39].
Epigastric pain, the presence of apparent submucosal vasculature and GERD were independently associated with resistant cases, to our knowledge, no study in our region was found to detect similar results. Persistent epigastric pain, pan gastritis, and associated duodenitis may reflect higher bacterial load and more severe disease, the increased load, especially among females, causes the decreased response to antibiotic therapy [17]. Despite the number of resistant females was higher than males (n=62 F vs. 30 M), value was not significant.
GERD was an independent feature of resistant cases; this may again raise the question if GERD in Egyptian population linked to H. pylori. We agreed with opinion of WU JC et al., [32] who explained this association through that H. pylori-positive patients had significantly lower basal Lower Esophageal Sphincter (LES) pressure, distal peristalsis amplitude, Ineffective oesophageal motility and oesophageal peristalsis failure which all play a dominant role in the development of GERD in these patients.
Lastly, another surprising finding in current study that patients with false negative H. pylori stool antigen either before treatment or following eradication therapy in patients with persistent symptoms prevalence was 22.8%. These patients found to have different endoscopic features as mentioned before, raise the question about the use of this test for diagnosis and confirmation of eradication of H. pylori in the locality.
Emara et al [20] also found, 26.8% of chronic active gastritis, 47.9% of positive H. pylori in histology, pan gastritis in 88.7% and nodularity in 12.7% in the group of negative H. pylori stool antigen Egyptian patient. The difference in the percentage of endoscopic pan gastritis was due to the inclusion of other patients with inactive gastritis and other causes of gastritis which were in exclusion criteria.
Limitation
Results of patients received different eradication therapies without endoscopic examination are not included for more precise detection of the resistant rate in Egyptian populations. Equal sampling of males and females is needed as this study included all patients referred to endoscopy regardless of sex in the determined duration. Larger studies are needed over longer periods to confirm association of demographic, clinical and endoscopic features previously reported with antibiotic resistance and to confirm its presence or absence with other lines of eradication therapy as identification of these features may help clinicians in referring these patients to susceptibility tests.
Conclusion
In present study, middle-aged female patients had more infection and referral for endoscopic evaluation. Persistent epigastric pain is the most common presentation. Pan gastritis is the common endoscopic criteria while mucosal nodularity was present in 21.8% especially in females. Mild activity is the more pronounced histological finding. Associated features as the presence of apparent submucosal vasculature, erosions, ulcers, hyperplastic polyps, incompetent cardia, GERD mainly of Type A and duodenitis were present. Resistant rate to first line therapy was 45.5% in patients referred to endoscopy. Epigastric pain, the presence of apparent submucosal vasculature and GERD have independently associated with resistant infection.
Qualitative data were represented as frequencies and relative percentages
Qualitative data were represented as frequencies and relative percentages
Chi-square test (χ2) and Fisher exact was used to calculate the difference between qualitative variables
Qualitative data were represented as frequencies and relative percentages
Chi-square test (χ2) and Fisher exact was used to calculate the difference between qualitative variables
β: regression coefficient; SE: standard error; OR: odds ratio; 95%CI: 95% confidence interval; p<0.05 is significant;
Forward multivariate logistic regression analysis model was done using any predictor with p<0.05 in the univariate analysis.
Qualitative data were represented as frequencies and relative percentages