In recent years, the association of Metabolic Syndrome (MS), which is a series of conditions including Insulin Resistance (IR), obesity, hypertension, and hyperlipidaemia, with malignancy attracted more attentions. As inevitable consequence of IR, hyperinsulinemia plays a crucial role in occurrence and prognosis of cancer [1].
High fasting serum insulin was associated with significantly poorer survival and disease-free survival in patients with early HCC [2].
RBP4 has gained much attention after the first notion that its serum level was enhanced in insulin resistant humans. Preclinical studies demonstrated that RBP4 might be a key regulator of IR and a crucial inducer of hyperinsulinemia [3].
Glucose Transporter (GLUT4) expression is decreased in adipocytes in nearly all insulin-resistant states in humans and rodents, but the mechanism by which this contributes to systemic insulin resistance has not been clear. It now seems that elevated serum RBP4 might be a mechanistic link by which down regulation of GLUT4 in adipocytes contributes to the development or worsening of systemic insulin resistance. It was found that RBP4 elevation is a widespread abnormality in insulin-resistant states of various aetiologies. Serum levels and/or urinary excretion of RBP4 have previously been reported to be elevated in humans with Type 2 diabetes, but no causal relationship was suggested [3].
Accumulating evidence showed that RBP4 exert a pivotal function to enhance pathogenesis in fatty liver disease and liver cirrhosis [4,5]. Recently attention has been paid to the role of RBP4 in the pathogenesis of IR. This protein, a member of the lipocalin family, is secreted mainly by hepatocytes (80%), but also by adipose tissue (20%). It is the only specific transport protein for retinol and, by interacting with nuclear Retinol X Receptor (RXR), it takes part in the control of metabolic and proliferative cell functions, including steatogenesis. Mouse and human studies have highlighted a pathogenic link among IR, diabetes, and high serum and adipose levels of RBP4, identifying RBP4 as a novel adipocytokine. There is clinical evidence that circulating RBP4 levels are related to the severity of IR and to the various features of the metabolic syndrome. Specifically, raised serum levels of RBP4 have been linked to non alcoholic fatty liver disease [5]. However, limited number of studies was conducted to investigate the relationship between RBP4 and HCC.
The role of RBP4 in liver cirrhosis has to be further investigated as it may act as a novel biomarker for screening of HCC.
The current study aimed to determine the value of serum RBP4 level in patients with HCV related liver cirrhosis and HCC and to correlate this level with the metabolic profile in these patients.
Materials and Methods
This study was conducted on 90 adult Egyptian patients (sample size determined using precision-based sample size calculations to estimate unknown parameter with the confidence level of 95%, z=1.96; assuming the population is normally distributed) with HCV (genotype 4) related liver cirrhosis (diagnosis based on clinical, biochemical and radiological investigations and classified according to Calculation of Child-Turcotte-Pugh score [6,7].
Patients were randomly recruited between October 2016 and November 2017 from the Hepatology outpatients’ clinic of Ain Shams University Hospitals.
Patients were excluded from the study if they had any of the following conditions: diabetes mellitus, current or past history of alcohol use, present or past history of any other malignant diseases other than HCC, organ transplant recipients, patients receiving hypolipidemic drugs, patients who underwent any form of bariatric surgery, patients on steatosis inducing drugs (i.e., corticosteroids, tamoxifen, amiodarone and valproic acid), intravenous drug users, patients with HIV infection, patients who received any form of treatment for HCC, obese patients (BMI>30), patients with other hepatic diseases as alcoholic liver disease, non alcoholic fatty liver disease, drug-induced hepatitis, other viral hepatitis, hereditary haemochromatosis, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis and alpha-1 antitrypsin deficiency.
Ninety patients were divided into two groups as follow:
Group I: Sixty patients with HCV related liver cirrhosis and HCC; diagnosis of HCC was based on the appearance of typical vascular pattern of enhancement in triphasic spiral CT scan of the abdomen.
Group II: Thirty patients with HCV related liver cirrhosis (HCC was excluded in these patients at the time of enrollment in the study; exclusion of HCC was based on the absence of any hepatic focal lesion in repeated abdominal ultrasonography scanning).
All patients were subjected to the following:
History taking, clinical examination, calculation of BMI (BMI=weight in kilogram/height in meters2) [7], abdominal ultrasonography scan, laboratory investigations including: complete blood picture, liver function tests, fasting and two hours post prandial blood glucose level, fasting serum insulin level, kidney function tests, fasting triglycerides and cholesterol levels, HBsAg, HCV and HIV antibodies using 3rd generation ELISA technique, serum alpha-fetoprotein level, prothrombin time and INR.
Calculation of Child-pugh score and MELD score: Calculations of MELD score using the following formula:
MELD=(0.957×log (creatinine)+0.378×log (bilirubin)+1.12×log (INR)+0.643)×10 (6 is the minimum score and >40 is the maximum score) [8] [Table/Fig-1].
Calculation of Child-Turcotte-Pugh score [7].
| Parameter | Points assigned |
|---|
| 1 | 2 | 3 |
|---|
| Ascites | Absent | Mild | Moderate-Tense |
| Serum bilirubin | <2 mg/dL | 2-3 mg/dL | >3 mg/dL |
| Serum albumin | >3.5 gm/dL | 2.8-3.5 gm/dL | <2.8 gm/dL |
| Prothrombin time (seconds over control) | <4 | 4-6 | >6 |
| INR | <1.7 | 1.7-2.3 | >2.3 |
| Encephalopathy | None | Grade 1-2 | Grade 3-4 |
Child Score: A=5-6, B=7-9, C=10-15.
INR: International neutralisation ratio
Oral Glucose Tolerance Test (OGTT) was done for all patients. Patients meeting the American Diabetes Association (ADA) criteria for diagnosis of diabetes mellitus in 2011 [9], Impaired Fasting Glucose (IFG), Impaired Glucose Tolerance (IGT) were excluded from this study. The test was performed as described by the World Health Organisation, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. Criteria for the diagnosis of diabetes: Fasting Plasma Glucose (FPG) >126 mg/dL or 2-h plasma glucose > 200 mg/dL during OGTT. IFG: FPG 100-125 mg/dL, IGT: 2-h plasma glucose in the 75 gm OGTT 140-199 mg/dL [9].
Insulin resistance was calculated using HOMA-IR index: HOMA-IR=fasting glucose (mmol/dL)×fasting insulin (gU/mL)/22.5 [10]. Patient was considered to have insulin resistance when HOMA-IR>2.5 [11,12].
Serum RBP4 level was measured by quantitative sandwich ELISA kits (Quantikine, R&D Systems, Minneapolis, MN) according to the manufacturer’s protocols.
All procedures performed in this study were in accordance with the ethical standards of Ain Shams University Research Committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Statistical Analysis
Data was analysed with the program SPSS under windows version 11.0.1. The statistical tests were included, calculation of the Mean values and standard deviation, Student’s t-test (t), Pearson Correlation coefficient (r) test, chi-square test (χ2), the Probability of error (P) was expressed as following: p-value >0.05: non-significant, p-value <0.05: significant, p-value <0.01: highly significant.
Sensitivity, specificity, diagnostic efficiency, Positive and Negative Predictive Values (PPV, NPV) and accuracy were calculated as shown in [Table/Fig-2].
Calculation of sensitivity and specificity.
| Reference standard | Reference standard | Formula |
|---|
| Self report | True Positive (TP) | False Positive (FP) | PPV=TP/(TP+FP) |
| Self report | False Negative (FN) | True Negative (TN) | NPV=TN/(FN+TN) |
| Sensitivity=TP/(TP+FN) | Specificity=TN/(FP+TN) | Accuracy=(TP+TN)/all cases examined |
The overall diagnostic performance of a test was assessed by (ROC) curve analysis
Results
A randomised, cross-sectional, comparative study was conducted on 90 Egyptian patients with HCV related liver cirrhosis. They were divided into two groups as follow: Group I included 60 patients with HCV related liver cirrhosis and HCC; there were 47 (78.3%) male and 13 (21.7%) female patients, Group II included 30 patients with HCV related liver cirrhosis with 19 (63.0%) male and 11 (36.7%) female patients.
The differences between the two groups regarding demographic data (gender, age, BMI) were statistically insignificant (p=0.129, 0.519 and 0.645 respectively).
The statistical differences between both the groups regarding all laboratory investigations are shown in [Table/Fig-3].
Comparison between two groups as regards laboratory investigations.
| Variables | Group I | Group II | t | p-value |
|---|
| Haemoglobin | Range | 5.300-14.300 | 7.800-13.800 | 0.406 | 0.686 |
| Mean±SD | 10.348±1.853 | 10.187±1.620 |
| White blood cell count | Range | 2.000-15.000 | 2.700-12.000 | 0.794 | 0.429 |
| Mean±SD | 6.820±3.102 | 6.316±2.205 |
| Platelets | Range | 32.000-394.000 | 27.000-427.000 | -0.356 | 0.723 |
| Mean±SD | 96.850±54.153 | 102.100±85.245 |
| ALT | Range | 10.000-307.000 | 10.000-169.000 | 0.616 | 0.540 |
| Mean±SD | 54.850±41.096 | 49.733±27.533 |
| AST | Range | 3.000-781.000 | 7.000-191.000 | 0.309 | 0.758 |
| Mean±SD | 71.217±98.113 | 65.533±31.764 |
| INR | Range | 1.100-4.700 | 1.200-5.400 | -1.480 | 0.142 |
| Mean±SD | 2.028±0.823 | 2.327±1.048 |
| Serum albumin | Range | 1.300-4.000 | 1.700-4.200 | -1.580 | 0.118 |
| Mean±SD | 2.500±0.601 | 2.707±0.550 |
| Total bilirubin | Range | 0.600-24.000 | 0.700-11.000 | 1.256 | 0.212 |
| Mean±SD | 4.079±3.816 | 3.140±2.077 |
| Direct bilirubin | Range | 0.120-16.000 | 0.140-6.000 | 0.946 | 0.347 |
| Mean±SD | 2.362±2.423 | 1.907±1.462 |
| Creatinine | Range | 0.500-3.700 | 0.400-3.400 | -0.985 | 0.327 |
| Mean±SD | 1.233±0.621 | 1.385±0.811 |
| Sodium | Range | 111.000-137.000 | 110.000-145.000 | -1.650 | 0.102 |
| Mean±SD | 127.467±5.950 | 129.833±7.264 |
| Potassium | Range | 2.800-5.700 | 3.000-5.900 | -3.842 | 0.100 |
| Mean±SD | 4.045±0.618 | 4.603±0.710 |
| Total Cholesterol | Range | 85.000-230.000 | 105.000-264.000 | -2.277 | 0.025** |
| Mean±SD | 146.033±37.368 | 165.667±40.868 |
| HDL | Range | 20.000-67.000 | 30.000-66.000 | -2.055 | 0.043** |
| Mean±SD | 40.317±11.588 | 45.533±10.862 |
| TG | Range | 35.000-185.000 | 62.000-195.000 | -3.029 | 0.003*** |
| Mean±SD | 102.733±40.215 | 129.567±38.357 |
| LDL | Range | 40.000-170.000 | 43.000-175.000 | -0.926 | 0.357 |
| Mean±SD | 87.617±28.874 | 94.133±36.228 |
| RBP4 (μg/mL) | Range | 8.500-150.000 | 6.000-60.000 | 2.572 | 0.012** |
| Mean±SD | 39.667±28.026 | 25.200±17.932 |
| HOMA-IR | Range | 0.400-7.300 | 0.370-5.160 | 2.180 | 0.032** |
| Mean±SD | 2.838±1.827 | 2.004±1.445 |
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International neutralization ratio; AFP: Alpha-feto protein; HDL: High density lipoprotein; LDL: Low density lipoprotein; TG: Triglycerides, HOMA: Homeostasis model of assessment
Patients with HCC had significant higher levels of serum RBP4 [Table/Fig-3]. However, neither the number of hepatic focal lesions nor the total tumour burden had any significant correlation with serum RBP4 levels [Table/Fig-4,5]. The distribution of hepatic focal lesions was as follows: 31 patients had single focal lesion, 15 patients had two focal lesions, 7 patients had three focal lesions and 7 patients had >3 focal lesions.
Correlation between serum RBP4 level and number hepatic focal lesions.
| Hepatic | RBP4 (μg/mL) | ANOVA |
|---|
| Focal lesion number | Range | Mean±SD | F | p-value |
|---|
| 1 | 12.000-150.000 | 37.726±27.400 | 0.955 | 0.440 |
| 2 | 8.500-95.000 | 40.467±24.843 |
| 3 | 12.000-110.000 | 56.071± 39.636 |
| 4 | 12.500-75.000 | 33.083±23.430 |
| 5 | 12.500-12.500 | 12.500±0 |
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant
Correlations between serum RBP4 level and laboratory investigations and total size of hepatic focal lesions.
| Laboratory investigations | RBP4 (μg/mL) |
|---|
| Group I | Group II |
|---|
| R | p-value | r | p-value |
|---|
| Child score | 0.131 | 0.320 | 0.135 | 0.477 |
| AFP | 0.170 | 0.193 | -0.177 | 0.350 |
| ALT | 0.042 | 0.752 | -0.011 | 0.954 |
| AST | -0.018 | 0.894 | -0.092 | 0.630 |
| INR | -0.033 | 0.801 | 0.030 | 0.875 |
| Serum albumin | 0.088 | 0.502 | -0.230 | 0.221 |
| Bilirubin | 0.093 | 0.480 | 0.286 | 0.125 |
| Cholesterol | -0.133 | 0.312 | -0.042 | 0.826 |
| HDL | -0.133 | 0.312 | -0.010 | 0.960 |
| TG | -0.183 | 0.161 | -0.051 | 0.788 |
| LDL | -0.116 | 0.377 | -0.035 | 0.856 |
| BMI | 0.090 | 0.493 | -0.010 | 0.959 |
| HOMA-IR | 0.128 | 0.330 | -0.015 | 0.936 |
| MELD score | 0.047 | 0.720 | 0.099 | 0.604 |
| Total size of hepatic focal lesions | 0.170 | 0.193 | 0 | 0 |
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant;
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International neutralization ratio; AFP: Alpha-feto protein; HOMA: Homeostasis model of assessment; HDL: High density lipoprotein; LDL: Low density lipoprotein; TG: Triglycerides; MELD: Model for End Stage Liver Disease
Serum RBP4 had insignificant correlations with all of the following: all laboratory investigations, Child score, MELD score, HOMAI-IR and BMI [Table/Fig-5,6].
Comparison between patients group regarding Child-Pugh score.
| Child-Pugh scoreGroup | Group I | Group II | |
|---|
| N | % | N | % | χ2 | p-value |
|---|
| A | 6 | 10.00 | 1 | 3.33 | 1.731 | 0.421 |
| B | 22 | 36.67 | 10 | 33.33 |
| C | 32 | 53.33 | 19 | 63.33 |
| Total | 60 | 100.00 | 30 | 100.00 |
N=number, %=percentage
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant.
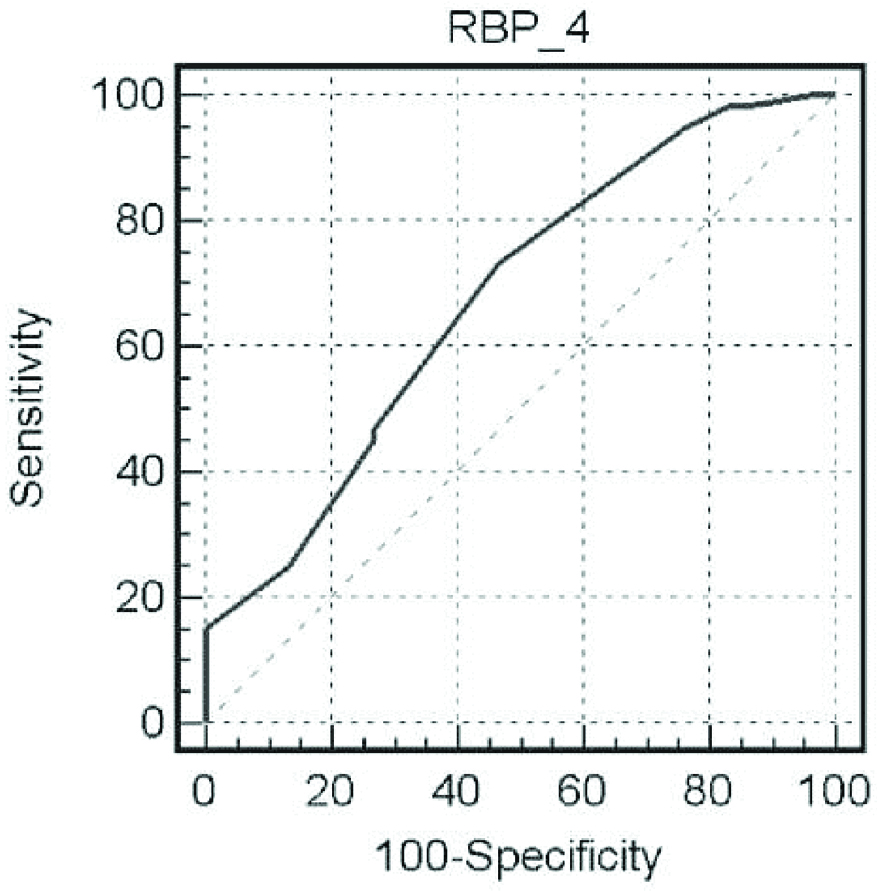
Serum RBP4 levels at cut-off value >12.5 μg/mL had 73.3% sensitivity and 53.3% specificity for detection of HCC (with an overall accuracy of 67.6%). Area under the ROC curve was equivalent to 0.676 [Table/Fig-7].
ROC curve analysis for RBP4 in detection of HCC.
Notably, higher values of HOMA-IR index were found among patients with HCC [Table/Fig-3].
Discussion
Hepatocellular carcinoma is the most common type of liver neoplasm. The incidence of HCC has increased by 80% in the last two decades in the United States [13]. This phenomenon was also observed in many of the developed countries [14]. HCC affects patients with chronic liver disease who have established cirrhosis, and currently is the most frequent cause of death in these patients. The major risk factors for its development are hepatitis B and C virus infection and alcoholism [15].
Chronic HCV infection is associated with the development of hepatic steatosis and virus-specific alterations in host metabolism leading to the development of IR [16], which is one of the worst metabolic disturbances of human body [17].
Liver is the major source of RBP4 in human body. Petta S et al., confirmed the previously recognised association of elevated serum RBP4 level with non alcoholic fatty liver disease [5]. Nevertheless, Yagmur E et al., suggested that patients with cirrhosis had a significantly lower serum RBP4 levels compared with healthy controls due to reduced hepatic biosynthetic capacity [18,19]. The focus of this study was to determine the value of serum RBP4 level in patients with HCV-4 related liver cirrhosis and HCC.
In this study, patients with HCC had a higher male-to-female ratio. This finding was in agreement with Hung CH et al., who stated that men are more susceptible to HCC than women [7]. This may be due to the fact that men are more likely to be infected with HBV and HCV, consume alcohol, smoke cigarettes, and have increased iron storage. Non environmental endogenous factors that may increase male risk include higher body mass index and higher levels of androgenic hormones [20].
The current study showed insignificant differences between patients with HCC and patients with liver cirrhosis as regards blood picture, liver function and kidney function tests. However, statistically significant differences were found between the two groups as regards total cholesterol, HDL and triglycerides levels. These results were in agreement with the results of Jiang J, et al., which stated that in the majority of HCC reports’, plasma levels of triglycerides, cholesterol, free fatty acids, HDL, LDL, lipoprotein (a), apolipoprotein AI and apolipoprotein B were slight to significantly decreased [21]. Morsy KH et al., reported significantly lower HDL levels in cirrhotic patients with HCC than in cirrhotic patients without HCC [22].
The present study found the mean serum values of RBP4 to be significantly higher in cirrhotic HCC patients than in cirrhotic patients without HCC with mean values of 39.7 pg and 25.2 pg respectively. These findings show an interesting phenomenon of re-upregulation of serum RBP4 level in patients with HCC from the low serum levels in patients with cirrhosis. Similar findings were shown by Wang DD et al., they reported significant higher serum RBP4 levels in patients with HCC (median=33.1 pg/mL) than in patients with liver cirrhosis (median=16 pg/mL) [23].
The current study also found serum RBP4 levels to be insignificantly correlated with both the number of tumour foci and the total tumour size. In contrast, Wang DD et al., reported significant higher serum RBP4 levels among patients with large tumour size and/or advanced disease stage [23].
Many studies showed that insulin may play an important role in carcinogenesis [24-26]. Insulin enhances carcinogenesis by activating IGF-I, hybrid insulin/IGF-I receptors, or other circulating factors involved in MS [27]. In accordance with the previous reports, the present study revealed a statistically significant higher mean HOMA-IR index among patients with HCC.
The significant higher serum values of RBP4 which was found in patients with HCC together with the insignificant correlation between serum RBP4 and HOMA-IR strongly points to the role of RBP4 in the malignant progression of HCC rather than in the induction of carcinogenesis which in turn may be more importantly related to circulating insulin levels. Also, RBP4 may be indirectly involved in induction of carcinogenesis as it was identified as a key upstream regulator of IR and inducer of elevated circulating insulin levels in obesity and in patients with diabetes [3,28]. The associations of serum RBP4 with tumour size, venous invasion, and TNM stage, elaborated in Wang DD et al., strongly indicate that serum RBP4 was involved in the malignant progression of HCC and confirms the results and the conclusion of the current study regarding the role of RBP4 in the malignant progression of HCC [23]. However, whether RBP4 stimulates carcinogenesis by insulin-IGF pathway was never confirmed.
Yao-Borengasser A et al., suggested that RBP4 is strongly associated with inflammation, especially infiltrating macrophages [29]. Another study reported high infiltrating macrophages in HCC tissue to be an important factor in accelerating HCC recurrence [30]. Moreover, RBP4 expression was found to be positively correlated with CD68 (specific marker of resident macrophage) expression. RBP4 was also found to induce IL-6 and TNF-a by the TLR4/NF-kB pathway which in turn mediates the activation of macrophages [31]. Therefore, it was proposed that RBP4 may promote HCC progression by means of macrophage activation [21].
As acknowledged by Wang DD et al., the finding of significant difference of serum RBP4 level between patients with HCC and cirrhotic patients without HCC suggested that serum RBP4 has the potential to be a biomarker for screening of HCC in patients with liver cirrhosis [23]. Further prospective studies are needed to assess the potential role of RBP4, at different cut-off values, biomarker for screening of HCC in patients with liver cirrhosis.
Wang DD et al., went further as they correlated the RBP4 level with the metastatic potential of HCC cell lines and the malignant phenotype of HCC cell. They also evaluated the fasting serum C-peptide level (a more stable marker of insulin exposure) and HOMA-IR in patients with HCC and found insignificant correlations of both with the overall survival and disease free survival intervals. In contrast, serum RBP4 had significant impacts on both the overall survival and disease free survival intervals. These findings raise the question if the underlying links between RBP4 and HCC are beyond the insulin-IGF axis. Moreover, serum RBP4, together with venous invasion and TNM stage, was identified as an independent risk factor of prognosis of patients with HCC [23].
Limitation
The present study had a few limitations. First, neither the hepatic pathological changes (stage of fibrosis, degree of necroinflammation and steatosis) nor the parameters of the metabolic syndrome were evaluated and were not correlated with serum RBP4 levels. Second, the number of patients included in the present study is relatively small. Third, the underlying, direct and indirect, links between RBP4 and HCC were not studied.
Conclusion
Retinol Binding Protein 4 was significantly re-upregulated in patients with HCC from its reduced levels in cirrhotic patients. RBP4 has the potential to be a new biomarker for screening of HCC in patients with liver cirrhosis.
Child Score: A=5-6, B=7-9, C=10-15.
INR: International neutralisation ratio
The overall diagnostic performance of a test was assessed by (ROC) curve analysis
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International neutralization ratio; AFP: Alpha-feto protein; HDL: High density lipoprotein; LDL: Low density lipoprotein; TG: Triglycerides, HOMA: Homeostasis model of assessment
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant;
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International neutralization ratio; AFP: Alpha-feto protein; HOMA: Homeostasis model of assessment; HDL: High density lipoprotein; LDL: Low density lipoprotein; TG: Triglycerides; MELD: Model for End Stage Liver Disease
N=number, %=percentage
p-value >0.05: non-significant, p-value <0.05**: significant, p-value <0.01***: highly significant.