Spinal Cord Injury (SCI) is defined as a serious damage to the spinal cord which primarily results from a sudden mechanical trauma to the spinal cord, leading to axonal disruption, tissue shearing and stretching, and cell death. In few hours and days after the injury, extensive neural cell degeneration is induced in the damaged tissue by a series of harmful secondary biological processes, including oxidative stress, calcium release, glutamate toxicity, and inflammation (neuroinflammation) [1, 2]. The neuroinflammation is known as a main contributing factor in the second phase of SCI as research has shown that modulating the inflammatory replies can ameliorate the SCI pathology and improve the functional recovery [3].
The NLR family, pyrin domain-containing protein 3 (NLRP3) inflammasome plays an important role in initiation of inflammation. The NLRP inflammasomes consist of a central scaffold protein (NLRP receptor, e.g., NLRP3), an adaptor protein apoptosis associated speck-like protein containing a caspase activation and recruitment domain (ASC), and precursor of the enzyme caspase-1 (pro-caspase-1). In response to danger signals released by damaged cells, the inflammasome components assemble which induces the activation of caspase-1. The activated caspase-1 regulates the maturation of pro-interleukin-1b (pro-IL-1β) and pro-IL-18 to generate IL-1β and IL-18, initiating the inflammatory cascades [4,5]. It has been shown that neurons and glia cells can produce and secrete IL-1β in distinct situations [6]. Besides, inflammasomes are assembled and activated in Central Nervous System (CNS) injury [6,7].
Anti-inflammatory characteristics of Mesenchymal Stem Cells (MSCs) have been focused in many studies, and researchers have utilised their therapeutic capacity in a wide range of inflammatory diseases [8,9]. MSCs transplantation has been reported that protects the CNS against different harmful insults through secretion of immunosuppressive factors, among other mechanisms [10].
The notes mentioned above persuaded us to investigate the efficacy of MSCs for inactivation of inflammasome complex. For this purpose, we measured the gene and protein expression of NLRP3 receptor in the spinal cord and concentration of IL-1β and IL-18 in serum following intracisternal transplantation of MSCs in SCI rats.
Materials and Methods
General Notes
This study was (with 28 rats) conducted during a three-month period (from October to December, 2017) and was approved by Ethical Committee of Tehran University of medical sciences (IR.TUMS.REC.1395.2278).
Animals and Grouping
Male wistar rats (260-280 g) were purchased from Animal Facilities of Institute of Biochemistry and Biophysics of Tehran University and maintained in the air-conditioned clean rooms (23°C±2) with 12:12 h light-dark alternative cycles and free access to water and food. Four random groups of animals (n=7) were designed as following: in group one the laminae were removed (laminectomy group), the spinal cord of animals in group two was injured after laminectomy (SCI group), animals in group three (vehicle group) and group four (WJMSCs group) underwent SCI operation and, 48 hours later, received 10 μL Phosphate Buffer Saline (PBS) or 3×105 Wharton’s Jelly Mesenchymal Stem Cells (WJMSCs), respectively.
Cell Culture
By explant method, WJMSCs were isolated from human umbilical cord Wharton’s jelly of newborns under informed consent of their parents. The jelly was obtained and chopped into 2-mm pieces inside sterile pellets containing DMEM/F12 medium. The pieces were plated in 25 cm2 cell culture flasks and fed with fresh DMEM/F12 medium supplemented with 10% Fetal Bovine Serum (FBS), 100 μg/mL streptomycin, and 100 units/ml penicillin (all from Gibco). The flasks were incubated in 5% CO2 incubator at 37°C and the medium was refreshed every three or four days. Approximately, after 10 days, the WJMSCs derived out from the jelly, attached to the floor, and expanded. WJMSCs at passage three with 80-90% confluency were used for flow cytometry and transplantation.
Flow Cytometry
The expression of two MSCs-specific markers (CD73 and CD90) and two haematopoietic stem cells- specific markers (CD34 and CD45) were evaluated in the cultured cells by flow cytometric test. Cells were treated with trypsin-EDTA (0.25%, Gibco, USA) for 5 minutes at 37°C, washed, suspended in PBS, and labeled with 1 μL of Fluorescein Isothiocyanate (FITC) or Phycoerythrin (PE)-conjugated monoclonal antibodies against the markers mentioned above (from EXbio, Czech Republic) for 30 minutes at room temperature. Then, the cells were washed, resuspended in PBS, and counted by a FACSCalibur Flow Cytometer (BD Biosciences, San Jose, USA).
Spinal Cord Surgery and Cell Transplantation
Anaesthesia was induced by a single dose of ketamine (80 mg/kg) and xylazine (15 mg/kg). The rats were fixed on a frame, the skin was incised, the muscles were dissected, and laminectomy was carefully performed at T10 level. To induce compressive SCI model, a 50 g weight rod bar with an area of 2.0×4.0 mm on the tip was placed on the exposed duramater for 5 minute in the groups two, three, and four. At the end, the incision was sutured and the animals were kept in separate cages until specimen removal at the day 21 after the surgery. The BBB locomotion test [11] was done on the next day after surgery and animals with a BBB score less than four were included in the study.
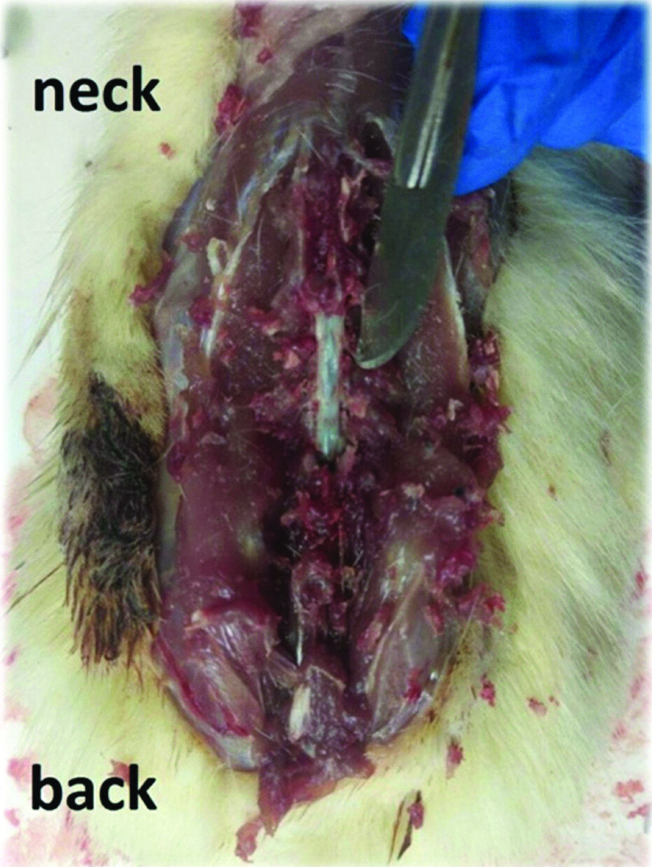
Under general anaesthesia, the animals were fixed on a board at the end of a worktable as their lumbar vertebral column was flexed. Then, the skin was shaved and incised longitudinally over L4-L5. Approximately, 3×105 WJMSCs were suspended in 10 μL PBS and injected into the lumbar cistern using a Hamilton syringe (Bonaduz, Switzerland) through the space between the laminae of L4 and L5. Cell delivery procedure was confirmed by injection of toluidine blue dye into the lumbar cistern of three extra rats and then detection of the dye in higher levels of subarachnoid space on the day after injection [Table/Fig-1]. The tail flick sign was considered as a confirmation of the needle insertion into the subdural space through dura mater.
Diffusion of toluidine blue dye in the subarachnoid space up to the thoracic level confirmed the intracisternal injection technique.
Real Time-PCR
The spinal cord specimens corresponding to 5 mm rostral and caudal to the injury site were freshly removed for real time-PCR assay (n=4). Total RNA was isolated from the specimens using phenol/guanidine-based QIAzolLysis Reagent (USA). Total RNA was used to supply complementary DNA (cDNA) using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to manufacturer’s guideline. Forward and reverse PCR primers were designed as listed in [Table/Fig-2]. Reactions were run in triplicate using StepOnePlus Real-Time PCR System (Applied Biosystems). Normalisation to GAPDH and calculation of relative fold change expression of NLRP3 was done by 2-∆∆Ct method [12].
| Gene | Forward primer | Reverse primer |
|---|
| NLRP3 | TCTTGAGGTCCACATCTTCC | TTTTCATTCCTGCTCTGCC |
| GAPDH | AAGTTCAACGGCACAGTCAAGG | CATACTCAGCACCAGCATCACC |
Western Blot Assay
Spinal cords 5 mm rostral and caudal to the injury site were obtained (n=3), lysed with RIPA buffer, and then their protein content was measured using the Bradford technique with Total Protein Kit, Micro (sigma, USA). The total protein was denaturised and loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and, next, transferred onto a nitrocellulose membrane (sigma, USA). The membrane was blocked by 5% dry milk in Tris-Buffered Saline (TBS) containing 0.05% Tween 20 (TBS-T) and then stained with primary antibody against NLRP3 (4°C overnight, Novus Biologicals, USA). On the next day, the membrane underwent incubation with secondary antibody conjugated with horse radish peroxidase (abcam, Germany) at 37°C for 1 hour. Lastly, the protein bands were visualised by luminescent substrate solution (sigma, USA), normalised to GAPDH, and analysed using software Image J (provided by NIH, USA).
Enzyme-linked Immunosorbent Assay (ELISA)
Animals were anaesthetised and phlebotomized with scalp needle into test tube. For serum derivation, the blood were left for 30 minutes at room temperature to coagulate and then centrifugated at 2000 rpm for 10 minutes. The sera were analysed by ELISA kits for rat (Diaclone SAS, France) to calculate the levels of IL-1β, IL-18 (three replicas) according to the manufacturer’s protocol.
Statistical Analysis
The data (mean±SD) were compared between different groups using one-way ANOVA followed by Tukey’s test in SPSS 22 software. A p-value less than 0.05% was considered significant.
Results
Identification of WJMSCs by Morphologic and Immunophenotypic Criteria
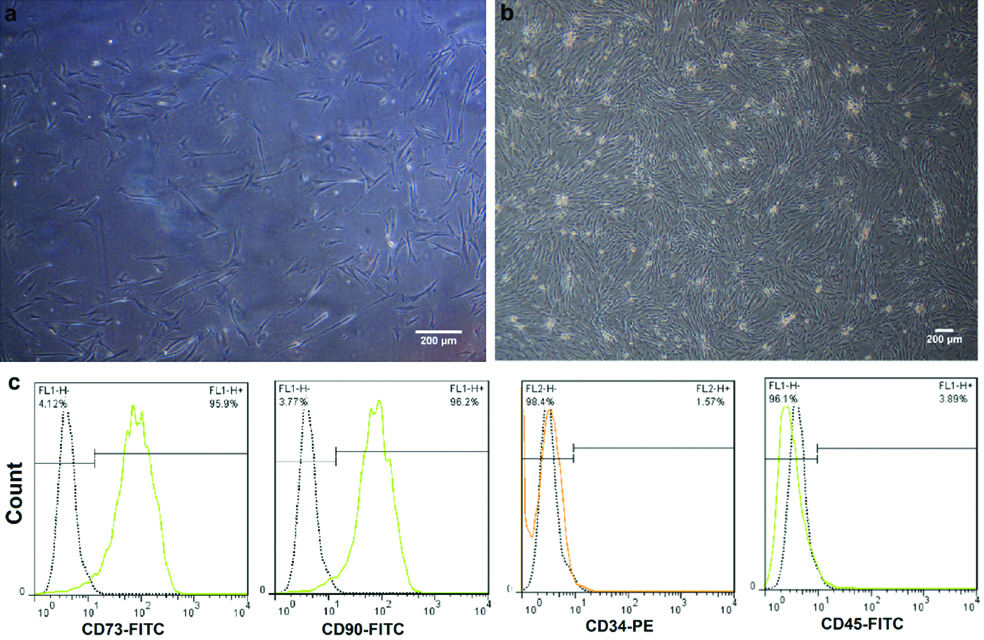
WJMSCs showed adhering feature, fibroblastic spindle appearance, and a confluence up to 90% after two to three weeks of culture [Table/Fig-3a,b]. In addition, flow cytometric test that was performed to assess the immunophenotypic features of the stem cells revealed high expressions of CD73 and CD90. On the contrary, the expression of CD34 and CD45 was inconsiderable [Table/Fig-3c]. These expression trends confirmed the mesenchymal identity of the cultured cells.
Morphologic and immunophenotypic features of WJMSCs. Phase contrast microscopic photos represent fibroblast-like morphology of WJMSCs (a) and their confluence (b). The pattern of CD marker expression in WJMSCs obtained from flow cytometry assay, which shows high expressions of CD73 and CD90 and low expressions of CD34 and CD45 (c).
Effect of WJMSCs on the NLRP3 Gene Expression
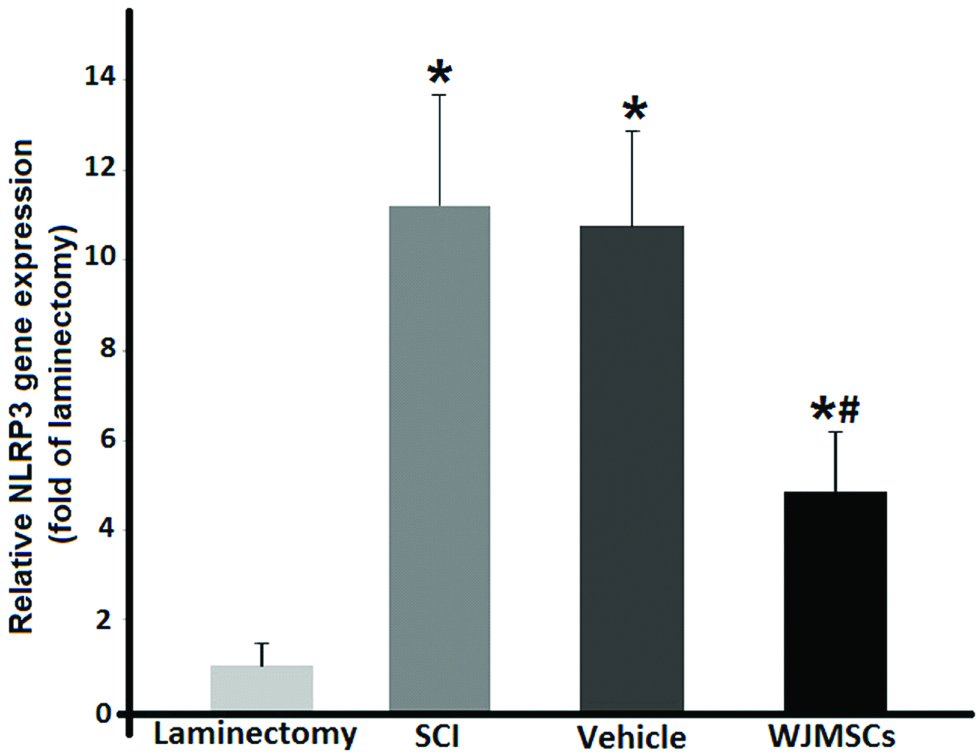
The injury significantly (p<0.05) increased the NLRP3 transcript level in the SCI group compared to the laminectomy group. It was found that WJMSCs had inhibitory effects on the expression of NLRP3 gene as it showed a significant decline in the treated rats in comparison to the laminectomy group (p<0.05). There was no significant difference between the means of mRNA level in the SCI and vehicle groups. Fold change expressions relative to the laminectomy group are seen in [Table/Fig-4].
mRNA levels of the NLRP3 gene in the SCI rats following treatment with intracisternal injection of WJMSCs.* and # p<0.05 vs. the laminectomy group. Data represent mean±SD.
Effect of WJMSCs on the NLRP3 Protein Expression
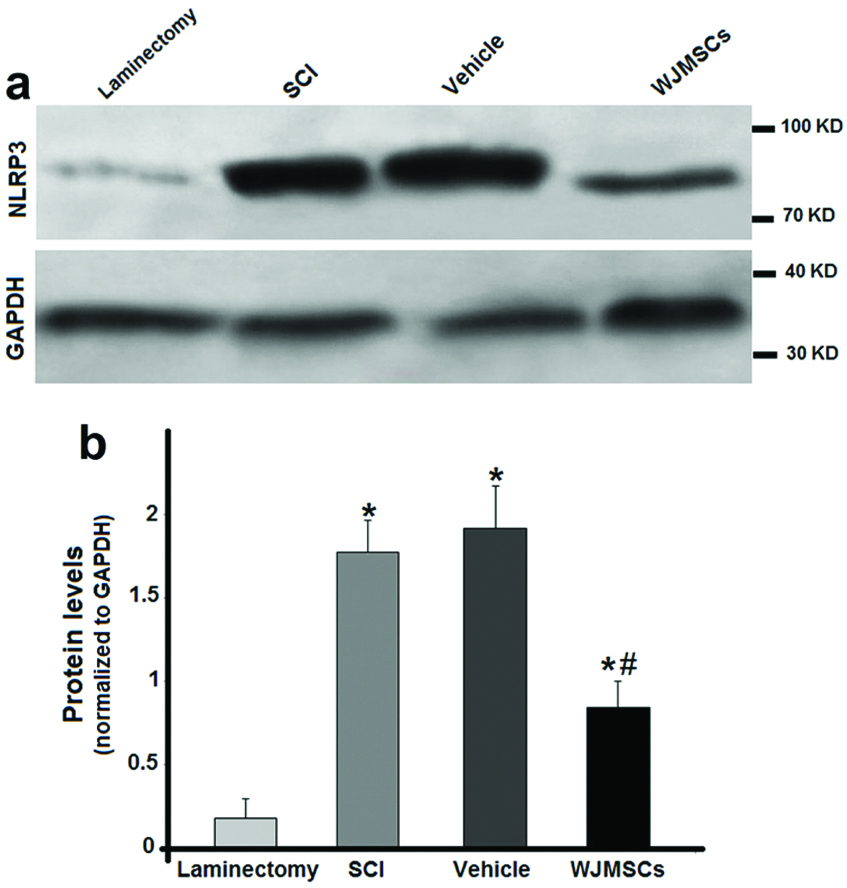
The effect of WJMSCs transplantation on the NLRP3 protein synthesis was determined by western blot assay [Table/Fig-5]. Based on the results, NLRP3 protein level showed an increase in the SCI group compared with the laminectomy group (p<0.05). SCI-dependent over expression of this protein was significantly decreased following WJMSCs injection, whereas the vehicle group did not have significant difference in comparison to the SCI group with regard to the NLRP3 protein.
The effect of intracisternal WJMSCs transplantation on protein level of the NLRP3 receptor after SCI. Western blots of NLRP3 (a) and their quantitative comparison between different groups (b) * and # p<0.05 vs. the laminectomy group. Data represent mean±SD.
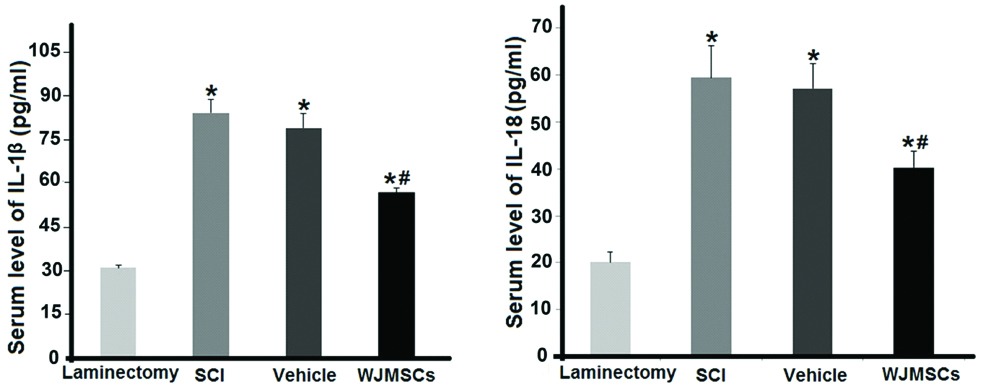
Effect of WJMSCs on the Levels of IL-1β and IL-18
Serum concentration of IL-1β and IL-18 was measured by ELISA assay and compared between different groups [Table/Fig-6]. Levels of these cytokines had a significant upward trend in the SCI group compared with the laminectomy group (p<0.05). ELISA assay indicated that WJMSCs transplantation was able to reduce significantly (p<0.05) the IL-1β and IL-18 concentrations in the treatment group in comparison to the SCI group. PBS injection in the vehicle group did not change the cytokines level compared to the SCI animals (p>0.05).
Serum concentration of IL-1β and IL-18 after WJMSCs transplantation in the SCI rats.* and # p<0.05 vs. the laminectomy group. Data represent mean±SD.
Discussion
Data indicate that WJMSCs reduce gene and protein levels of the NLRP3 domain of the inflammasome complex in the injured spinal cord of rat. In addition, it is consistent with the NLRP3 expression trend, the changes in the serum concentration of IL-1β and IL-18 revealed that WJMSCs diminish their secretion, as well. We think that the inhibition of NLRP3 production by WJMSCs may be a mechanism for decline in the level of these pro-inflammatory cytokines. However, this notion needs to be more investigated using NLRP3 antagonists.
The inflammasomes are caspase-1 activators which play an important role in triggering inflammatory cascades. They have a receptor to sense the disruption of cell homeostasis and to identify danger signals released by injured cells. Extracellular ATP and ROS are considered as danger signals for activation of the NLRP3 inflammasome [13]. It has been demonstrated that the NLRP3 inflammasome is activated in the CNS after both acute disorders such as ischemia [14] and chronic inflammatory diseases such as Alzheimer’s disease [7]. Using double-immunofluorescence staining of the injured spinal cord, Zendedel A et al., showed that the NLRP3 protein is mainly expressed by neurons [15]. However, microglia and astrocytes may contribute to the NLRP3 expression. Efforts for inhibiting inflammasome formation have resulted in better recovery in different CNS diseases. Inflammasome gene silencing in cerebral ischemic stroke [16], stromal cell-derived factor-1 (SDF-1) and oestrogen administration in SCI [15,17], and utilising a synthetic inhibitor in Alzheimer’s disease [18] are some examples of targeting the inflammasome complex for therapeutic purposes.
SCI has a bad prognosis and in spite of different endeavors from traditional medicine [19] to platelet-rich plasma injection [20] there is no effective treatment for this crippling injury. Cell therapy has been seriously focused as a great promise for treating SCI. The mechanisms by which trauma impairs the spinal cord tissue should be considered when a novel treatment is developed. Treatment with MSCs has been considered as a hopeful strategy in CNS diseases because of multiple beneficial features of these stem cells, such as differentiation into neuronal cells, neuroprotective, and neuroregenerative properties [21,22]. One of the main capacities of MSCs which motivates researchers to utilise them for SCI treatment is their immune modulatory effect. Immunosupressive secretory products of MSCs attenuate the inflammation aspect of injuries through reduction of inflammatory cytokines [23]. Because the inflammasome activation occurs upstream of the production of inflammatory cytokines, It was thought that MSCs may influence the SCI-induced neuroinflammation by inhibition of the NLRP3 inflammasome. Previously, an in vitro study investigated the effect of MSCs on the activity of NLRP3 inflammasome in macrophages. It demonstrated that the expression of inflammasome components in lipopolysaccharide- or ATP-stimulated macrophages was reduced following the addition of MSCs to the culture [24]. Here, this work was set out to investigate the inhibitory potential of WJMSCs on the inflammasome in spinal cord-injured rats, in vivo. It was found that intracisternal transplantation of WJMSCs declined the expression of NLRP3 domain of the inflammasome in both gene and protein levels. In addition, serum analysis by ELISA indicated that the levels of IL-1β and IL-18 which are produced downstream to the inflammasome activation were decreased after transplantation of WJMSCs. Based on the in vitro studies, the inhibitory effects of MSCs on the inflammasome formation have been attributed to secretion of stanniocalcin-1 anti-apoptotic protein and prostaglandin E2 [24,25]. The exact interaction of MSCs with the inflammasome complex needs to be elucidated in the future.
In the current study, it was demonstrated that WJMSCs has ability to suppress the expression of the inflammation trigger on NRLP3 receptor in the SCI rats. Detection of danger signal by receptor is the first step of inflammasome activation. This event results in caspase-1 recruitment by ASC protein that is necessary for secretion of the pro-inflammatory interleukins [4]. This study did not cover the effect of WJMSCs transplantation on the expression of ASC and caspase-1 mediators. We intend to investigate this hypothesis in the near future and believe that it would introduce a more concise description of inflammasome inactivation via WJMSCs.
Conclusion
Based on the results, intracisternal transplantation of WJMSCs inhibited the expression of NLRP3 receptor and also reduced the secretion of inflammatory cytokines IL-1β and IL-18 after SCI. Therefore, it can be concluded that MSCs modulate the neuroinflammation from its beginning step by preventing the formation of inflammasome complex.