Thyroid gland is the first endocrine glandular structure to be differentiated and start to develop about 24 days after fertilization [1]. The thyroid hormones are necessary for regulating the basal metabolic rate, somatic growth, psychic growth, calcium metabolism and circadian rhythm [2]. Hypothyroidism is the most common disorder of the endocrine system seen in approximately 1 in 3500 children and affects the normal psychosomatic growth [3].
The development of foetal thyroid function is dependent on the embryogenesis, differentiation and maturation of the thyroid gland. The serum Thyroid Stimulating Hormone (TSH) is detectable at 12 weeks and peaks maximum between 18-22 weeks [4].
The development of thyroid gland is coupled with evolution of the hypothalamic-pituitary-thyroid axis and thyroid hormone metabolism, resulting in the regulation of thyroid hormone action, production and secretion [5].
In a normal physiological state, the gland histology presents with round or oval follicles. The bigger follicles in the peripheral and smaller ones are situated in the central zone of the gland. The walls of the follicles are lined with one layer of cuboidal epithelium. This fluctuates in height based on the functional status of the gland. In the lumen of the follicles there is an eosinophilic, homogenous colloid, containing resorptive vacuoles [6,7].
The functional activity of thyroid gland can be correlated with structural changes in thyroid gland. Hence the present study was undertaken to assess the histogenesis of thyroid gland by noting various changes in the capsule, size of peripheral and central follicles, appearance of colloid and amount of colloid in thyroid follicles of dead human Foetuses of different gestational age.
Materials and Methods
This was a cross-sectional study. Forty spontaneously aborted dead human Foetuses with gestational age ranging from 11 weeks to 38 weeks were collected from Bharati Vidyapeeth Medical College and Hospital, Sangli and other private hospitals with the kind permission of the concerned authorities, consent of parents and Ethical committee approval number BVDUMC&H/Sangli/IEC/Dissertation/2013-14/66. The Foetuses were collected over the period of four years (From Oct. 2013 to Oct. 2017). Dead aborted and still born normal human Foetuses were included. Twins and Foetuses with gross congenital anomalies as well as mothers having metabolic or hormonal disorder were excluded. The gestational age was estimated by using Crown Rump Length (CRL) and foot length confirmed by referring Last Menstrual Period (LMP). These Foetuses were divided into six groups according to their gestational age [Table/Fig-1].
Groups according to Gestational Age.
| Groups | Crown Rump length (cm) | Foot length (cm) | Gestational age | Number of Foetuses |
|---|
| I | 7.5-11.5 | 0.8-1.9 | 11-13 Weeks | 03 |
| II | 12.5-14.7 | 2.2-2.5 | 14-16 Weeks | 03 |
| III | 15.7-18.8 | 3.0-3.9 | 17-20 Weeks | 14 |
| IV | 19.5-24 | 4.2-5.2 | 21-25 Weeks | 12 |
| V | 25-30 | 5.8-6.5 | 26-32 Weeks | 05 |
| VI | 31-38 | 6.8-7.5 | >33 Weeks | 03 |
The fixation of the Foetuses was done by injecting 5% formalin locally at various sites with the help of 10 mL syringe and needle in abdominal, thoracic, cranial cavity and subcutaneously in the upper and lower limbs. After injecting formalin, Foetuses were kept in 5% buffered formalin filled glass jars for 10 days before dissection.
The Foetuses were dissected by making midline incision on the anterior aspect of neck. The infrahyoid muscles were separated and the bi-lobed thyroid gland was removed. The tissue slices of 3-4 mm thickness were taken and fixed with Bouins fluid for 24 hours. The tissue was passed through graded alcohol (50%, 70%, 80%, 90% and absolute alcohol) for dehydration. Clearing was done by using two changes of xylene at 1 hour each. Embedding of tissue was done in paraffin wax for two changes (melting point 56°C) of one hour each. The paraffin blocks were made by using L moulds. The sections were cut at 5 μm thickness by using rotary microtome. The ribbons of sections were thrown on the surface of warm water in tissue floating bath then sections were taken on slides coated with egg-albumin. Slides were kept for drying on a hot plate at 40°-50° C for 2 hours or more as per requirement.
Staining Procedure
PAS stain: PAS satin was used for colloid confirmation in the Foetuses of group I and group II. Paraffin was removed with xylene followed by graded alcohol (100%, 90%, 70% and 50%). Then the sections were oxidized with periodic acid for 5 minutes and then rinsed in several changes of distilled water. The sections were covered with Schiff reagent for 15 minutes and then rinsed under running tap water for 5-10 minutes. Heamatoxylin was used to stain nuclei followed by bluing. Sections were washed with alcohol, cleaned with xylene and mounted with DPX.
Haematoxylin and Eosin: Paraffin was removed with xylene followed by graded alcohol (100%, 90%, 80%, 70% and 50%). Then the sections were stained with haematoxylin solution. Excess stain was removed by dipping into acid alcohol for a few seconds followed by bluing. Counter staining was done with eosin. Sections were washed with alcohol, cleared with xylene and mounted with DPX.
The slides were observed under light microscope and the following points were noted:
Histogenesis of thyroid gland as precolloid stage, colloid formation stage, folliculogenesis stage and secretory activity stage along with development of the capsule.
Size of the follicle was measured by using stage micrometer and eyepiece reticle at 45x magnification. The line of micrometer scale coinciding with line of micrometer eyepiece was observed and the value of equidistant line was decided. On calculation, 1 division of micrometer eye piece was 4 μm. By using this scale diameters of peripheral thyroid follicles (A) and central thyroid follicles (B) were calculated at 10xs by 40xs.
The nature, amount of colloid content of the follicle and stroma at different stages of development.
The sections were observed under a CX21i Biological microscope and the images were captured with Olympus CCD camera (U-TV0.5XC-3). Processing of images was done with Magnus Pro 3.7 software and analysed.
Results
Histology of thyroid gland was studied at different gestational age:
Photomicrograph showing thyroid gland with precolloid stage, at 13th weeks under 45X, (2a-H&E) and (2b-PAS). Arrow shows cords and nests or clusters F - Follicle.
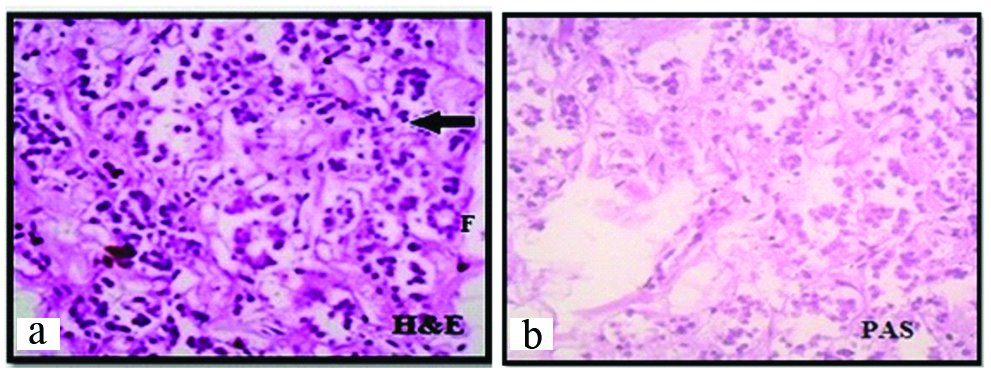
The gland was covered by thin capsule. In this group, the centre of the gland showed large number of clusters and cords of epithelial cells (Arrow). At some places oval to round follicles were observed [Table/Fig-2a]. The lining epithelium was cuboidal with darkly stained nucleus. At the periphery of the gland, 30-35 μm diameter follicles were noted. Colloid was not found at this stage which was confirmed by PAS staining [Table/ Fig-2b]. So this is precolloid stage.
Photomicrograph showing H&E and PAS staining of thyroid gland with colloid stage, at 14th weeks under 10X (a); and 45X (B&C). Arrow shows: 1) Capsule; 2) Septa with blood vessels; 3) Clusters and cords of epithelial cells; 4) Rim of colloid material.
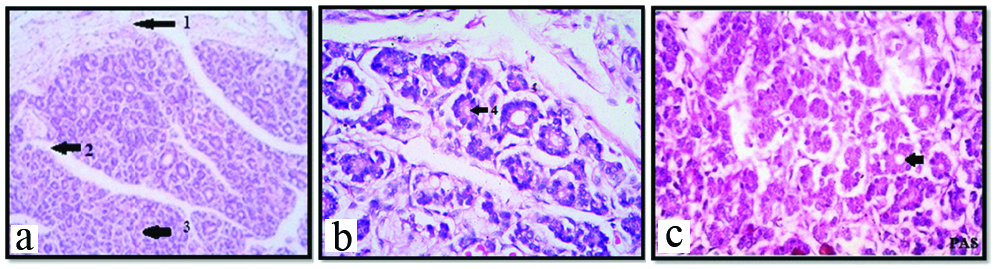
The capsule of gland showed increase in thickness and vascularity. The septa divided the gland into incomplete lobules. Along with septa blood vessels invaded the gland. The follicles showed progressive differentiation inwards from periphery of the gland [Table/Fig-3a]. The central core of the gland still contained clusters and cords of epithelial cells and very few follicles were seen. The shape of follicles was round to oval surrounded by rich network of blood vessels. Follicular cells were simple cuboidal with darkly stained nuclei. Thin rim of colloid was present in few developing follicles [Table/Fig-3b,c]. Sinusoids were present between the follicles. PAS staining showed PAS positive colloid in peripheral follicles [Table/Fig-3c]. At the periphery of the gland, the diameter of the follicles was 40-60 μm and that of central follicles was 20-40 μm. This is colloid formation stage.
Photomicrograph showing thyroid gland with folliculogenesis stage, at 20th week under 10X (a); and 45X (b) respectively (H&E). Arrow shows, Parafollicular or C cells.
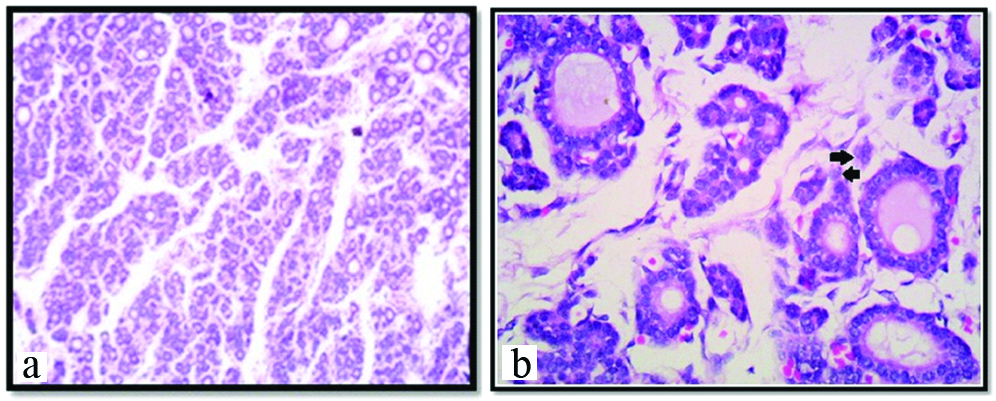
In this stage, capsule was well developed with increased vascularity. The peripheral and central core of the gland showed increased number of follicles than previous stage. In the central core of the gland, developing follicles were seen which were very small in size with or without a lumen. Sinusoids were abundantly present. Hence, this stage was considered as folliculogenesis stage [Table/Fig-4a]. Colloid was seen in almost all follicles but prominent in peripheral follicles with intrafollicular vacuoles [Table/Fig-4b]. The parafollicular cells or C cells were noted [Table/Fig-4b]. At the periphery of the gland, the diameter of the follicles was 60-115 μm and that of central follicle was 40-60 μm.
Photomicrograph showing thyroid gland with secretory stage, at 25th week under 10X (a); and 45X (b) respectively (H&E). Arrow shows: 1) Irregular eroded colloid with vacuoles; 2) Parafollicular or C cells
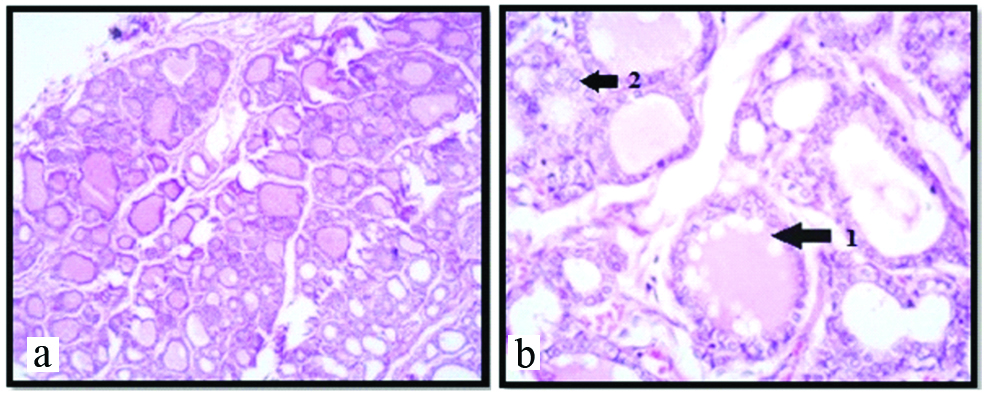
In this stage the capsule and septae were well defined. The thick connective septa along with blood vessels were seen invading the gland. More number of mature thyroid follicles was seen in the Foetuses of this group [Table/Fig-5a]. The epithelial cells were cuboidal with centrally placed nucleus. Vascularity was increased amongst the follicles. Some follicles showed irregular eroded colloid with vacuoles in it [Table/Fig-5b]. The presence of colloid and its affinity with acidic dye, the secretory activity of the gland is more at this stage of development [Table/Fig-5a]. Hence this stage was considered as secretory stage. At the periphery of the gland, the diameter of the follicles was 120-210 μm and that of central follicle was 80-100 μm. The parafollicular cells or C cells were noted [Table/Fig-5b].
Photomicrograph showing thyroid gland with increased vascularity at 30th weeks under 10X (a); and 45X (b) respectively (H&E). Arrow showing: 1) Thick capsule; 2) Septa with blood vessels; 3) Parafollicular cells or C cells.
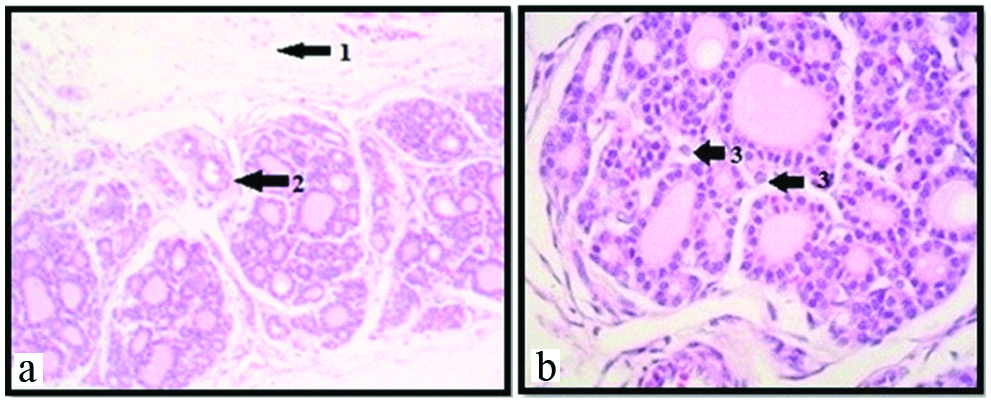
In this group, the thyroid gland show increased number and size of follicles with darkly stained colloid material [Table/Fig-6a]. In some follicles, colloid appeared irregular and eroded in the portion alongside the follicular cells. At the periphery of the gland, the diameter of the follicles was 120-220 μm and that of central follicle was 60-100 μm. The parafollicular cells or C cells were noted [Table/Fig-6b].
Photomicrograph showing the histological picture of the thyroid gland at 38th weeks under 10X (a); and 45X (b) respectively (H&E) similar to that of adult. Arrow shows parafollicular or C cells.

In this group, throughout the gland the vascularity was increased. Follicles were full of colloid material in the lumen. This stage shows increased synthesis as well as excretory activity of the gland. The histological picture of the thyroid gland was more or less similar to that of adult [Table/Fig-7a,b]. At the periphery of the gland, the diameter of the follicles was 110-250 μm and that of central follicle was 60-120 μm.
Discussion
At 12th week, the capsule was thin with small number of blood vessels but as age advances, the capsule became thick and its vascularity also increased. This observation was similar with the observation of human thyroid studies of Ham AW et al., [8].
According to Potter EL, during the development of human thyroid gland, initial cord like arrangement of epithelial cells gets organized to form small follicles which are filled with an acidophilic material called as colloid. At birth epithelial cells lining the follicle contain a small nucleus surrounded by moderate amount of colourless cytoplasm. The cytoplasm often bulges into the lumen of the gland, producing a scalloped margin for the colloid. The largest follicles are located at the periphery of lobule [9].
The differentiation of thyroid follicles started from the periphery of the gland and extended centrally, as the periphery of the gland is more vascular than the central part throughout the gestational age of Foetuses. These findings were similar to the observation reported by Potter EL [9].
Thin rim of colloid appeared in the developing follicles of 10-17 weeks Foetuses and increased as gestational age advanced [10]. Similar findings were observed in group II in this study.
Hamilton noted the follicular stage at 14th week of gestation or late. Bocian-sobkowska J et al., noted that, the stage of massive folliculogenesis and gradual accumulation of colloid appeared between 10th-18th week. Gaikwad JR observed that the folliculogenesis stage was more prominent between 14th-20th week stages of foetal thyroid. Anupriya A et al., observed that folliculogenesis was very well established between 11-22 weeks. In the present study, folliculogenesis stage was observed between 13th-20th week [6,10-12].
Mitskavitch MC observed that the presence of colloid in the follicle, its affinity with acidic dyes, clear vacuoles in the colloid. The apical position of nuclei is considered as indicative of the secretory activity of the follicular stage [13].
According to Gaikwad JR the secretory activity of the gland was more at 20th-24th week. Whereas, it was found that the secretory activity was more prominent between 20th-25th weeks [6].
Arthur WH reported that the colloid is often seen to have shrunken away from the follicular epithelium in such a way as to present a serrated rather than a smooth outline, when the gland is active [14]. this study correlated with above findings.
In present study from 25th week onward, the colloid appeared irregular and eroded at periphery along the apices of follicular cells of the thyroid gland. Similar observations were also noted by Junqueira IC et al., [15].
Shepard TH et al., observed that human foetal thyroid gland having same age group showed different stages of maturity of thyroid follicles; even mature follicles were not developed in some areas of full term Foetuses [7]. Such immature follicles were also seen in group V and VI Foetuses of this study especially in central region of the gland. Brown R et al., reported that full maturation of thyroid follicles occur at 36-40 week [16].
Hamilton divided the maturation of thyroid follicles into 3 stages:
The precolloidal stage- 7-13 weeks
The colloid formation stage –(65-80 mm CRL) – 13 to 14 weeks
The follicular stage (after 80 mm CRL) 14 wks onwards [10].
Limitation
The limitation of this study was the constricted range of gestational age of foetuses from 11th to 38th weeks. Hence, it cannot be commented how the early developmental changes must have appeared in the foetuses before 11 weeks.
Conclusion
In present study of histogenesis of thyroid gland, Precolloidal stage was seen before 13th week and linear but partially overlapping developmental stages like folliculogenesis, colloid formation and maturation of follicles were observed after 14th week of gestation. The present study would help the clinicians to understand the development and correlate with histopathological changes in certain thyroid gland disorders.