Introduction
MHO is a medical condition characterised by obesity without occurrence of any metabolic complications. Till date a universally acceptable definition for MHO is not yet established. It is in general agreement that, MHO though associated with obesity, lacks metabolic abnormalities such as dyslipidaemia, impaired glucose tolerance, diabetes, or metabolic syndrome. MHO individuals usually have less visceral adipose tissue, smaller adipocytes, and a reduced inflammatory profile when compared to metabolically unhealthy obese individuals and individuals with Metabolic Syndrome (MetS) [1-5].
Epidemiology: MHO as a term has not been satisfactorily defined. Individuals of such phenotypes as per various criterias applied exhibit an extreme degree of variability in the prevalence that ranges between 10-34% applying various definitions [6,7] As per the epidemiologic trends, the prevalence of MHO decreases with age in both genders and is more prevalent in women than in men. Individuals with normal weight also are at increased risk of developing the MHO [8,9].
Existing definitions: Separate studies define MHO in different ways. Some studies defined obesity as BMI ≥30 kg/m2. Some researchers defined obesity as either BMI ≥30 kg/m2 or body fat >25% in men and 30% in women. Some included measures of inflammation {either C-Reactive Protein (CRP) or White Blood Cell (WBC) count} in the definition of MHO. Others defined “metabolically healthy” as absence of MetS based on the Third Report of the National Cholesterol Education Program, Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults (NCEP ATP III); definition of MetS without any modification. Some studies defined “metabolically healthy” as absence of MetS with study specific modifications to the MetS criteria. The definition which required the simplest of criteria defined MHO as BMI 30 kg/m2 with less than three metabolic syndrome criteria [10-13].
Pathophysiology of MHO
1. Subclinical inflammation: Medical research has substantial evidence to suggest that it is the adipose tissue inflammation which plays as a major determinant if at all an individual will manifest a healthy metabolic obesity or otherwise. Subclinical adipose tissue inflammation most often resulting in insulin resistance can be best assessed by predictors like the CRP. One of the best predictors for assessing vascular inflammation, metabolic syndrome and cardiovascular diseases would be to analyse CRP levels in circulation [14]. Scientific assessment suggests CRP to be one of the best markers for assessing metabolic syndrome in young individuals as it correlates well with various anthropometric markers and body composition [15].
Lean individuals tend to have more of anti inflammatory adipokines in their blood. Examples of such adipokines are adiponectin, IL-4, IL-10, IL-13, IL-1 receptor antagonist, and transforming growth factor beta. Individuals with metabolic healthy phenotype usually tend to have low levels of certain important serological markers in comparison to Metabolically Unhealthy Obese (MUO) individuals. The markers present in MHO individuals at low levels include Complement C3, Alpha necrosis factor, CRP, Interleukin 6, WBCs in MHO individuals are also reduced suggesting a low inflammatory state within them. Individuals who are obese tend to have pro-inflammatory marker in circulation like leptin, resistin, TNF-α, IL-6, IL-18, retinol-binding protein 4, lipocalin 2, angiopoietin-like protein 2, CC-chemokine ligand 2, CXC-chemokine ligand 5, nicotinamide phosphoribosyltransferase, free fatty acids and activated leukocytes in circulation [16].
Obese individuals with type 2 DM definitely have high serological levels of IL6, FFA and activated leukocytes in comparison to obese and morbidly obese non-diabetic individuals [17-19]. Similarly, the serological markers seen in obese and morbidly obese subjects without metabolic disease in comparison to normal lean individuals was similar and there is no infiltration of macrophages and elevated values of TNF and IL-6 expression in visceral adipose tissue [20].
Above mentioned findings suggest that individuals with MUO in comparison to MHO have a much higher degree of adipose tissue inflammation both at systemic as well as local levels.
2. Adipose tissue expandability hypothesis: This hypothesis explains the transition of adipose tissue leading to a metabolic state. The hypothesis suggests that each adipocyte has its own capacity and once it reaches its threshold, inflammatory changes within the adipocyte starts taking place, this in turn produces insulin resistance, resulting in type 2 diabetes mellitus. Various scientific animal knockout studies carried out on mechanism of adipokine secretion, with Peroxisome Proliferator-Activated Receptor (PPAR) and lipodystrophic models have supported the adipose expandability hypothesis.
3. Genetic theory: Further studies have shown that many genetic contributors are involved in the process of adipogenesis, apoptosis and angiogenesis. The various genes and protein present in the visceral and subcutaneous tissue like PPARs, Diacylglycerol O-Acyltransferase 1, Aquaporin 7, insulin, and HOMA-IR has shown positive correlation between adipose tissue metabolism and insulin pathway. In these regulatory genes predispose to various patterns of obesity [21].
4. Adaptation hypothesis: Another significant strong theory particularly puts forth the concept that different subjects or individuals tend to have variable capacity in terms of adipose tissue storage and adaptation to excess energy. It is these attributes of an individual which pushes them either towards a metabolic health obesity or an unhealthy metabolic obesity [22]. Further the theory suggests that when storage of fat is required the adipose tissue can increase in size or number [23]. While expanding of the adipose tissue takes place it also requires vascularity [24], individuals having an uneventful expansion and increase in overall mass of adipose tissue (both cellularity as well as size of cells) remain healthy with obesity, conversely if an unhealthy and difficult expansion of adipocytes takes place it results in appearance of metabolic disease [25] [Table/Fig-1].
Pictorial image showing factors linked to MHO.
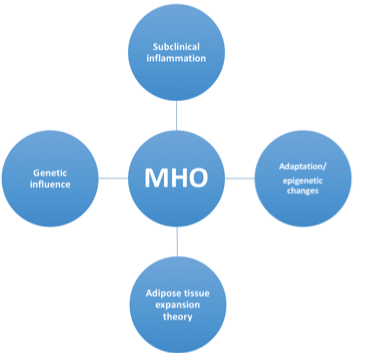
The theory also puts forth the concept that even in lean individuals where the adipose tissue expansion is unhealthy metabolic disease tends to develop which explains the existence of metabolic disease even in lean individuals or metabolic unhealthy lean subjects [26]. As the adipose tissue loses it capacity to expand and store fat, the adipocyte loses its lipogenic function and as a result there is also formation of lipotoxic substances which further damage the adipocyte and cause and promote inflammation [27].
Stem cells in form of preadipocytes behave as progenitor cells, they are found in the vascular stroma. The adipocyte tends to also vary depending in number depending on the cells undergoing apoptosis, necrosis [28-33]. Studies have also suggested fibrosis and stress as significant predisposing factors resulting in reduced number of adipocytes. Ultimately it can be said that the capacity of adipocyte functionality and expansion depends on certain important factors like ability of the adipocytes to undergo lipogenesis, adipogenesis from progenitor cells, programmed apoptosis of adipocytes, and angiogenesis to adipocytes [21].
Going along with aforesaid mentioned hypothesis; individuals with adipocytes which have good lipogenic and angiogenic capacity, have a healthy metabolic obesity in contrast to individuals with unhealthy metabolic obesity [34-36]. The mesenchymal cell capacity to metamorphose into new formed adipocytes is often present in metabolic healthy individuals. Such MHO individuals tend to express increased number of mesenchymal cells in the adipose tissue stroma with a high capacity to differentiate into bone and adipose tissue, have lower senescence. Further studies have also shown that there is increased apoptosis in the adipocytes along with increased inflammation in unhealthy metabolic obesity [37].
Metabolic syndrome, atherosclerosis, diabetes mellitus are all majorly linked to adipose tissue inflammation. Inflammation of adipose tissue is a key regulator for development of unhealthy metabolic obesity. Most of the studies point towards the fact that inflammation of an adipocyte is a prerequisite for development of unhealthy metabolic obesity, however further elaborate studies would help analyse the behaviour of inflammatory cytokines which promote effective adipocyte inflammation less or no inflammation promoting MHO and prominent inflammation by cytokines promote unhealthy metabolic obesity. The distribution of adipose tissue in the body mainly encompasses the adipose tissue of the peripheral subcutaneous areas as well as the systemic visceral adipose tissue deposits. The adipose tissue mainly affected by the metabolic disorders belongs to the visceral and intrahepatic areas; these areas tend to have a direct association with metabolic unhealthy obesity. Contrastingly the subcutaneous areas are minimally or almost never associated with metabolic obesity [38].
Considering the above mentioned adipose tissue storage areas; subcutaneous as well as visceral compartments, A more rational hypothesis suggests that healthy obesity occurs when the fat is deposited in the subcutaneous areas however when the capacity of fat deposition is reduced or lost in the subcutaneous tissue, the fat accumulation tends to deposit in other adipose tissue compartments like visceral and hepatic resulting in metabolic diseases [39]. Taking into consideration the above hypothesis the phenotype of MHO individuals may need to be evaluated and redefined as a group of obese individuals with a dominant subcutaneous distribution of fat and almost negligible visceral distribution, further search needs to be carried out for the same in order to categorise such individuals as MHO phenotypes.
1. MicroRNA’s/Adiponectin in metabolic obesity: Recent studies have pointed towards microRNAs as important potential factors which play a major role in development of metabolic obesity. The microRNA has also been implicated in development of increased peripheral resistance for insulin in organs like adipose tissue, liver and muscle. Hence, microRNA can behave as potential markers to differentiate MHO and MUO individuals [40].
The MUO individuals having high levels of circulating microRNAs contribute to metabolic obesity [41]. Studies have shown that individuals who are overweight (BMI over 40 kg/m2) tend to have serum adiponectin levels higher in comparison to individuals who have BMI within normal set limits. Adiponectin seems to be a potential marker for predicting metabolic health status, with individuals having adiponectin above certain level have higher probability of being metabolic healthy, taking into consideration the effects of age, insulin and waist circumference. Adiponectin plays a major role in development and pathogenesis of metabolic syndrome and their emanating complications.
Interestingly individuals with obesity and having a healthy metabolic phenotype have similar adiponectin levels as that of normal weight individuals. MHO individuals have a lesser amount of fatty tissue in the visceral and hepatic compartments in comparison of subcutaneous fat distribution. Most importantly, even the life style factors like exercise and physical activity play a major role in development of metabolic obesity, tilting balance either towards metabolic healthy and or unhealthy obesity. Researches show that MHO individuals have a better and active physical life style in comparison to MUO individuals [13,42].
Fate of MHO?
Debate continues regarding the true health risks of MHO. Current literature differs in regards to the relative risk of disease among this population. Though MHO individuals are at decreased risk for developing cardiovascular disease when compared with MUO individuals, still they have an increased risk of progressing to MetS in future. In a study involving a group of Japanese Americans with MHO, two-thirds developed metabolic syndrome during 10 years. Visceral fat accumulation, female sex, higher fasting plasma insulin concentration, and lower serum HDL were independently associated with MetS. In the Pizarra study, the prevalence of MHO decreased during 11 years of observation with conversion to MetS consistent with the hypothesis that MHO progresses to MetS [43].
A recent study evaluated the prevalence of elevated plasma high sensitivity CRP (hs-CRP) concentrations and hepatic steatosis in MHO, MHNW, and in MUNW individuals [44]. They observed that both elevated plasma hs-CRP concentrations and hepatic steatosis are more prevalent among MHO and MUNW individuals than they are among MHNW individuals. However, they are most prevalent among individuals with MHO, suggesting that obesity in the absence of metabolic risk factors is not entirely benign but is associated with subclinical vascular inflammation. Another recent study reported that 42% of their subjects with MHO developed the metabolic syndrome within 10 years [45], again suggesting that MHO is not without increased health risks.
MHO and Cardiovascular Disease Risk
Metabolic healthy obesity- the term safeguards obesity with evidence of absent cardiometabolic risk factors. However contrary to the belief, researches are now providing ample evidence that MHO individuals are at an increased risk of several adverse outcomes, including Type 2 diabetes and cardiovascular events. These novel researches argue that even if MHO individuals have a normal metabolic profile, this does not safeguard them from conventional cardiovascular risks and decrease mortality. Increasing evidence is now pointing that MHO individuals are at a higher risk of cardiovascular disease compared to metabolically healthy non-obese individuals, though when compared with MetS these risks are lower.
A recent meta-analysis evaluated eight studies comparing mortality data from any cause or cardiovascular events in six groups of patients defined by BMI category (n=61,386) and metabolic comorbidities, with a 10-year follow-up. The results of analysis depicted that MHO population had an increased risk compared with MHNW subjects, in 10 or more years of follow-up. Another study also published similar results indicating that both MHO and MUO individuals have a higher risk of mortality [46] [Table/Fig-2].
Illustrates different studies confirming the cardiovascular risk status in MHO [48-56].
| Hinnouho GM et al., [43] | MHO subjects were at increased risk for CVD (HR 1/4 1.97, 95% CI: 1.38-2.80) and type 2 diabetes (3.25, 95% CI: 2.32-4.54). |
| Khan UI et al., [46] | Cross-sectional analysis compared Common Carotid Artery Intima Media Thickness (CCA-IMT), aortic Pulse Wave Velocity (aPWV) and Coronary (CAC) and AorticCalcification (AC) in MHO.The study concluded that metabolically benign overweight/obese womenhave a significantly greater subclinical CVD burden than normal weight women. |
| Roberson LL et al., Meta analysis [47] | MHO was found to be significantly associated with all-cause mortality in (30%), CVD mortality in (14%), and incident CVD (33%) as compared to their MHNW counterparts. |
| Caleyachetty R et al., [48] | The study concluded that MHO individuals had a higher risk of coronary heart disease, cerebrovascular disease, and heart failure than normal weight metabolically healthy individuals. |
| Jung CH et al., [49] | MHO subjects had a significantly higher prevalence of significant subclinical coronary atherosclerotic burden compared with Metabolically Healthy Non-Obese (MHNO) subjects. |
| Kramer CK et al., [50] | The study concluded that when compared with metabolically healthy normal-weight individuals, obese persons are at increased risk for adverse long-term outcomes even in the absence of metabolic abnormalities, suggesting that there is no healthy pattern of increased weight. |
| Fan J et al., [51], meta analysis | Compared with healthy normal-weight individuals, MHOW and obese (MHOB) individuals showed increased risk for CVD events, which appeared much stronger during the long-term follow-up period of >15 years. |
| Zheng R et al., [52] | In this meta-analysis, the Relative Risk (RR) calculated on the basis of the incident number of disease events and deaths in MHO subjects. The meta-analysis confirmed apositive association between a metabolically healthy obese phenotype and the risk of CVD. |
| Gómez-Ambrosi J et al., [53] | Performed a cross-sectional analysis to compare the cardiometabolic/inflammatory profile of 222 MHO subjects.Expression of genes involved in inflammation and tissue remodeling in visceral adipose tissue and liver showed a similar alteration pattern in MHO and Metabolic abnormal obesity (MAO) individuals. |
| Kuk JL and Ardern CI [54] | Examined the risk for all-cause mortality in metabolically normal (MHO) andabnormal obese (MAOB) individuals.MHO subjects had similar elevations inmortality risk compared with metabolically normal, normal weight subjects.The study concluded that although a rare phenotype, obesity, even in the absence of overt metabolic aberrations, is associated with increased all-cause CVD mortality risk. |
| Ogorodnikova AD et al., [55] | Using pooled data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies, assessed incident CVD using three definitions of the metabolically healthy obesity.The CVD incidence rates (mean follow-up 11.8 years) were 7.1, 5.8, and 8.4 per 1,000 person-years in metabolically benign obese via the three definitions suggesting MHO is not a benign condition. |
| Brant LC et al., [56] | Meta-analysed aggregate data from 3 large cohorts (Brazilian Longitudinal Study of Adult Health, the Framingham Heart Study, and the Gutenberg Heart Study. Regression slopes between cardiovascular risk factors and microvascular function, measured by Peripheral Arterial Tonometry (PAT), were calculated in MHO. Metabolically healthy obese individuals had more impaired vascular function than metabolically healthy normal-weight individuals. |
Conclusion
There is now no paucity of data that emphasises that MHO is actually not a “healthy” phenotype. In fact, any obesity is bad. Therefore it is high time; we adjudge how healthy is MHO and do not remain in the trance of being in good health even if obesity is present without the conventional cardiovascular risk factors. As rightly said; MHO is the early “Snap-shot” of future pathway to metabolic syndrome and other cardiovascular risks. Rather “Premetabolic syndrome” may be a term that would replace “MHO” in time to come in the era of evidence based medicine.
[1]. Rishi P, Is it finally time to dispel the concept of metabolically-healthy obesity?Journal of the American College of Cardiology 2014 63(24):2687-88.10.1016/j.jacc.2014.03.04324794116 [Google Scholar] [CrossRef] [PubMed]
[2]. Häring SN, Hu HU, Schulze FB, Matthias B, Metabolically healthy obesity: epidemiology, mechanisms, and clinical implicationsThe Lancet Diabetes & Endocrinology 2013 1(2):152-62.10.1016/S2213-8587(13)70062-7 [Google Scholar] [CrossRef]
[3]. Denis GV, Obin MS, “Metabolically healthy obesity”: Origins and implicationsMolecular Aspects of Medicine 2013 34(1):59-70.10.1016/j.mam.2012.10.004 [Google Scholar] [CrossRef]
[4]. Navarro E, Funtikova AN, Fíto M, Schröder H, Can metabolically healthy obesity be explained by diet, genetics and inflammation?Molecular Nutrition & Food Research 2014 59(1):75-93.10.1002/mnfr.20140052125418549 [Google Scholar] [CrossRef] [PubMed]
[5]. Bluher M, Mechanisms in endocrinology: Are metabolically healthy obese individuals really healthy?European Journal of Endocrinology 2014 171(6):R209-R219.10.1530/EJE-14-054025012199 [Google Scholar] [CrossRef] [PubMed]
[6]. Van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studiesBMC Endocrine Disorders 2014 14(1):910.1186/1472-6823-14-924484869 [Google Scholar] [CrossRef] [PubMed]
[7]. Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH, The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions usedObes Rev 2014 15(10):781-90.10.1111/obr.1219825040597 [Google Scholar] [CrossRef] [PubMed]
[8]. Matthias B, The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individualsCurrent Opinion in Lipidology 2014 21(1):38-43.10.1097/MOL.0b013e3283346ccc19915462 [Google Scholar] [CrossRef] [PubMed]
[9]. Susann B, Peter S, Metabolically healthy obesity from childhood to adulthood- Does weight status alone matter?Metabolism 2014 63(9):1084-92.10.1016/j.metabol.2014.06.00925038727 [Google Scholar] [CrossRef] [PubMed]
[10]. Arnlov J, Ingelsson E, Sundstrom J, Lind L, Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged menCirculation 2010 121(2):230-36.10.1161/CIRCULATIONAHA.109.88752120038741 [Google Scholar] [CrossRef] [PubMed]
[11]. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular diseaseJ Clin Endocrinol Metab 2006 91(8):2906-12.10.1210/jc.2006-059416735483 [Google Scholar] [CrossRef] [PubMed]
[12]. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB, State of disparities in cardiovascular health in the United StatesCirculation 2005 111(10):1233-41.10.1161/01.CIR.0000158136.76824.0415769763 [Google Scholar] [CrossRef] [PubMed]
[13]. Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN, The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitnessEur Heart J 2013 34(5):389-97.10.1093/eurheartj/ehs17422947612 [Google Scholar] [CrossRef] [PubMed]
[14]. Sutherland J, McKinley B, Eckel R, The metabolic syndrome and inflammationMetab Syndr Relat Disord 2004 2:82-104.10.1089/met.2004.2.8218370640 [Google Scholar] [CrossRef] [PubMed]
[15]. DeBoer M, Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetesamong adolescents: A need for screening tools to target interventionsNutrition 2013 29:379-86.10.1016/j.nut.2012.07.00323022122 [Google Scholar] [CrossRef] [PubMed]
[16]. Phillips C, Perry I, Does inflammation determine metabolic health status in obese and nonobese adults?J Clin Endocrinol Metab 2013 98:E1610-19.10.1210/jc.2013-203823979951 [Google Scholar] [CrossRef] [PubMed]
[17]. Ouchi N, Parker JL, Lugus JJ, Walsh K, Adipokines in inflammation and metabolic diseaseNat Rev Immunol 2011 11:85-97.10.1038/nri292121252989 [Google Scholar] [CrossRef] [PubMed]
[18]. Van Beek L, Lips M, Visser A, Pijl H, Ioan-Facsinay A, Toes R, Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose toleranceMetabolism 2014 63:492-501.10.1016/j.metabol.2013.12.00224467914 [Google Scholar] [CrossRef] [PubMed]
[19]. Blüher S, Schwarz P, Metabolically healthy obesity from childhood to adulthood-does weight status alone matter?Metabolism 2014 63:1084-92.10.1016/j.metabol.2014.06.00925038727 [Google Scholar] [CrossRef] [PubMed]
[20]. Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, The obese healthy paradox: Is inflammation the answer?Biochem J 2010 430:141-49.10.1042/BJ2010028520522023 [Google Scholar] [CrossRef] [PubMed]
[21]. Muñoz-Garach A, Cornejo-Pareja I, Tinahones FJ, Does metabolically healthy obesity exist?Nutrients 2016 8(6):32010.3390/nu806032027258304 [Google Scholar] [CrossRef] [PubMed]
[22]. Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A, From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammationNutr Metab Cardiovasc Dis 2009 19:146-52.10.1016/j.numecd.2008.10.01019171470 [Google Scholar] [CrossRef] [PubMed]
[23]. De Ferranti S, Mozaffarian D, The perfect storm: obesity, adipocyte dysfunction, and metabolic consequencesClin Chem 2008 54:945-55.10.1373/clinchem.2007.10015618436717 [Google Scholar] [CrossRef] [PubMed]
[24]. Ledoux S, Queguiner I, Msika S, Calderari S, Rufat P, Gasc JM, Angiogenesis associated with visceral and subcutaneous adipose tissue in severe human obesityDiabetes 2008 57:3247-57.10.2337/db07-181218835936 [Google Scholar] [CrossRef] [PubMed]
[25]. Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Adipocyte turnover: Relevance to human adipose tissue morphologyDiabetes 2010 59:105-09.10.2337/db09-094219846802 [Google Scholar] [CrossRef] [PubMed]
[26]. Virtue S, Vidal-Puig A, It’s not how fat you are, it’s what you do with it that countsPLoS Biol 2008 6:e23710.1371/journal.pbio.006023718816166 [Google Scholar] [CrossRef] [PubMed]
[27]. Barbarroja N, Rodriguez-Cuenca S, Nygren H, Camargo A, Pirraco A, Relat J, Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and functionDiabetes 2015 64:1180-92.10.2337/db14-035925352638 [Google Scholar] [CrossRef] [PubMed]
[28]. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Dynamics of fat cell turnover in humansNature 2008 453:783-87.10.1038/nature0690218454136 [Google Scholar] [CrossRef] [PubMed]
[29]. Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissueFASEB J 2009 23:3494-505.10.1096/fj.08-12694619584303 [Google Scholar] [CrossRef] [PubMed]
[30]. Isakson P, Hammarstedt A, Gustafson B, Smith U, Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammationDiabetes 2009 58:1550-57.10.2337/db08-177019351711 [Google Scholar] [CrossRef] [PubMed]
[31]. Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM, IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjectsEndocrinology 2010 151:3752-63.10.1210/en.2010-004320555032 [Google Scholar] [CrossRef] [PubMed]
[32]. Roldan M, Macias-Gonzalez M, Garcia R, Tinahones FJ, Martin M, Obesity short-circuits stemness gene network in human adipose multipotent stem cellsFASEB J 2011 25:4111-26.10.1096/fj.10-17143921846837 [Google Scholar] [CrossRef] [PubMed]
[33]. Sorisky A, Gagnon AM, Clinical implications of adipose tissue remodelling: Adipogenesis and apoptosisCan J Diabetes 2002 26:232-40. [Google Scholar]
[34]. Ortega FJ, Mayas D, Moreno-Navarrete JM, Catalán V, Gómez-Ambrosi J, Esteve E, The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjectsObesity 2010 18:13-20.10.1038/oby.2009.20219543203 [Google Scholar] [CrossRef] [PubMed]
[35]. Clemente-Postigo M, Queipo-Ortuño MI, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones FJ, Cardona F, Adipose tissue gene expression of factors related to lipid processing in obesityPLoS One 2011 6:e2478310.1371/journal.pone.002478321966368 [Google Scholar] [CrossRef] [PubMed]
[36]. Cao Y, Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseasesNat Rev Drug Discov 2010 9:107-15.10.1038/nrd305520118961 [Google Scholar] [CrossRef] [PubMed]
[37]. Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levelsBMC Physiol 2012 12:410.1186/1472-6793-12-422471305 [Google Scholar] [CrossRef] [PubMed]
[38]. Tinahones FJ, Coín-Aragüez L, Murri M, Oliva-Olivera W, Mayas Torres MD, Barbarroja N, Caspase induction and BCL2 inhibition in human adipose tissue: A potential relationship with insulin signaling alterationDiabetes Care 2013 36:513-21.10.2337/dc12-019423193206 [Google Scholar] [CrossRef] [PubMed]
[39]. Bluher S, Markert J, Herget S, Yates T, Davis M, Muller G, Who should we target for diabetes prevention and diabetes risk reduction?Curr Diabetes Rep 2012 12:147-56.10.1007/s11892-012-0255-x22298028 [Google Scholar] [CrossRef] [PubMed]
[40]. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Circulating micro RNAs as stable blood-based markers for cancer detectionProc Natl Acad Sci 2008 105:10513-18.10.1073/pnas.080454910518663219 [Google Scholar] [CrossRef] [PubMed]
[41]. Zhang T, Lv C, Li L, Chen S, Liu S, Wang C, Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individualsBio Med Res Int 2013 2013:76161710.1155/2013/76161724455723 [Google Scholar] [CrossRef] [PubMed]
[42]. Katzmarzyk P, Church T, Janssen I, Ross R, Blair S, Metabolic syndrome, obesity, and mortality: Impact of cardiorespiratory fitnessDiabetes Care 2005 28:391-97.0.2337/diacare.28.2.39115677798 [Google Scholar] [CrossRef] [PubMed]
[43]. Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort studyEur Heart J 2015 36:551-59.10.1093/eurheartj/ehu12324670711 [Google Scholar] [CrossRef] [PubMed]
[44]. Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotypeInt J Obes 2015 39:1365-70.10.1038/ijo.2015.7525920773 [Google Scholar] [CrossRef] [PubMed]
[45]. Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, Garcia-Fuentes E, Almaraz MC, Colomo N, Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra studyJ Clin Endocrinol Metab 2013 98:2318-25.10.1210/jc.2012-425323559087 [Google Scholar] [CrossRef] [PubMed]
[46]. Khan U, Wang D, Thurston R, Sowers M, Sutton-Tyrrell K, Matthews K, Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The Study of Women’s Health Across the Nation (SWAN)Atherosclerosis 2011 217:179-86.10.1016/j.atherosclerosis.2011.01.00721310415 [Google Scholar] [CrossRef] [PubMed]
[47]. Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, Beyond BMI: The "Metabolically healthy obese" phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality- a systematic reviewBMC Public Health 2014 8:1410.1186/1471-2458-14-1424400816 [Google Scholar] [CrossRef] [PubMed]
[48]. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and womenJ Am Coll Cardiol 2017 70:1429-37.10.1016/j.jacc.2017.07.76328911506 [Google Scholar] [CrossRef] [PubMed]
[49]. Jung CH, Lee MJ, Hwang JY, Jang JE, Leem J, Yang DH, Association of metabolically healthy obesity with subclinical coronary atherosclerosis in a Korean populationObesity (Silver Spring) 2014 22(12):2613-20.10.1002/oby.2088325155902 [Google Scholar] [CrossRef] [PubMed]
[50]. Kramer CK, Zinman B, Retnakaran R, Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysisAnn Intern Med 2013 159(11):758-69.10.7326/0003-4819-159-11-201312030-0000824297192 [Google Scholar] [CrossRef] [PubMed]
[51]. Fan J, Song Y, Chen Y, Hui R, Zhang W, Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studiesInt J Cardiol 2013 168(5):4761-68.10.1016/j.ijcard.2013.07.23023972953 [Google Scholar] [CrossRef] [PubMed]
[52]. Zheng R, Zhou D, Zhu Y, The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a systematic review and meta-analysisJ Epidemiol Community Health 2016 70(10):1024-31.10.1136/jech-2015-20694827126492 [Google Scholar] [CrossRef] [PubMed]
[53]. Gómez-Ambrosi J, Catalán V, Rodríguez A, Andrada P, Ramírez B, Ibáñez P, Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthyDiabetes Care 2014 37(10):2813-21.10.2337/dc14-093725011950 [Google Scholar] [CrossRef] [PubMed]
[54]. Kuk JL, Ardern CI, Are metabolically normal but obese individuals at lower risk for all-cause mortality?Diabetes Care 2009 32:2297-99.10.2337/dc09-057419729521 [Google Scholar] [CrossRef] [PubMed]
[55]. Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP, Incident cardiovascular disease events in metabolically benign obese individualsObesity (Silver Spring) 2012 20:651-59.10.1038/oby.2011.24321799477 [Google Scholar] [CrossRef] [PubMed]
[56]. Brant LC, Wang N, Ojeda FM, LaValley M, Barreto SM, Benjamin EJ, Relations of metabolically healthy and unhealthy obesity to digital vascular function in three community-based cohorts: a meta-analysisJ Am Heart Assoc 2017 6(3):pii:e00419910.1161/JAHA.116.00419928275071 [Google Scholar] [CrossRef] [PubMed]