Acute Native Valve Endocarditis by Granulicatella adiacens
Suman Susan Prakash1, Deepashree Rajshekar2, Akshatha Ravindra3, R Sneha4, Apurba Sankar Sastry5
1 Junior Resident, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
2 Senior Resident, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
3 Junior Resident, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
4 Junior Resident, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
5 Associate Professor, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Apurba Sankar Sastry, Office of HICC, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry-605006, India.
E-mail: drapurbasastry@gmail.com
Infective Endocarditis (IE) is an uncommon but a life-threatening infectious disease. Approximately 3% to 5% of cases of streptococcal infective endocarditis is caused by Nutritionally Variant Streptococci (NVS). Granulicatella species is one of the rare causes of IE. Here, authors are reporting a case of native valve endocarditis, without any pre-existing cardiac lesions, caused by Granulicatella adiacens. The patient presented with low grade intermittent fever for three months and had pan-systolic murmur with hepatomegaly and multiple vegetations in the echocardiogram. All the blood cultures were positive for Granulicatella adiacens. The patient was successfully treated with intravenous ceftriaxone and gentamycin for six weeks.
Infective endocarditis, Life-threatening, Nutritionally variant streptococci
Case Report
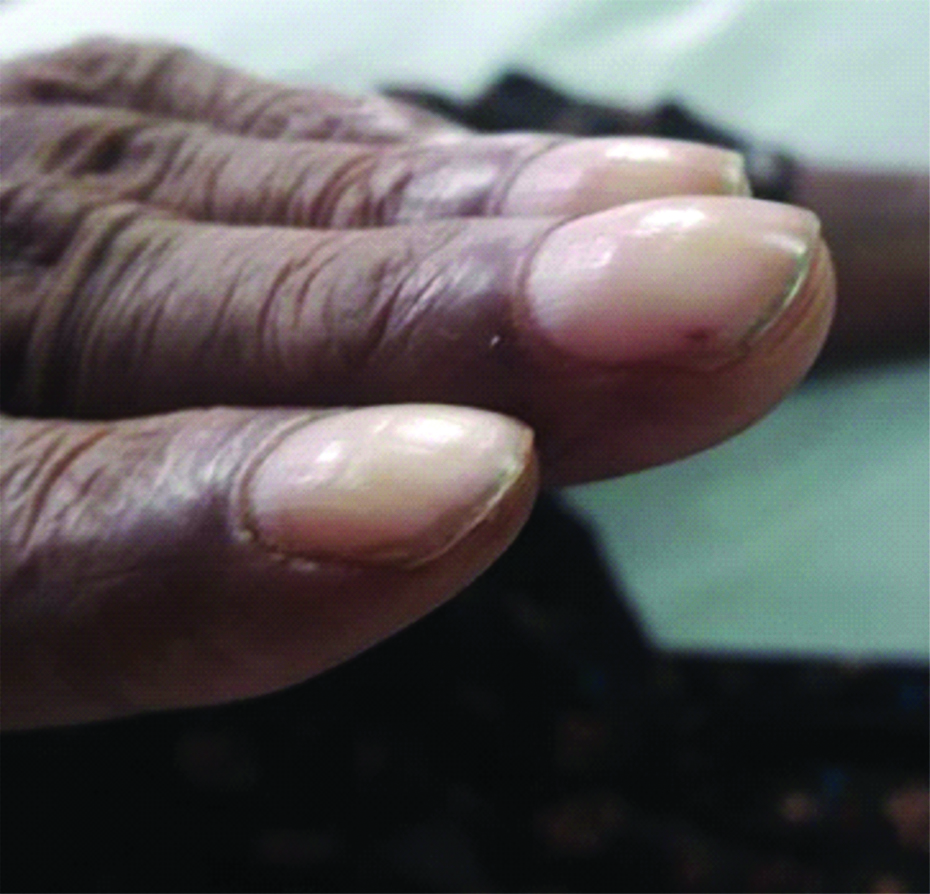
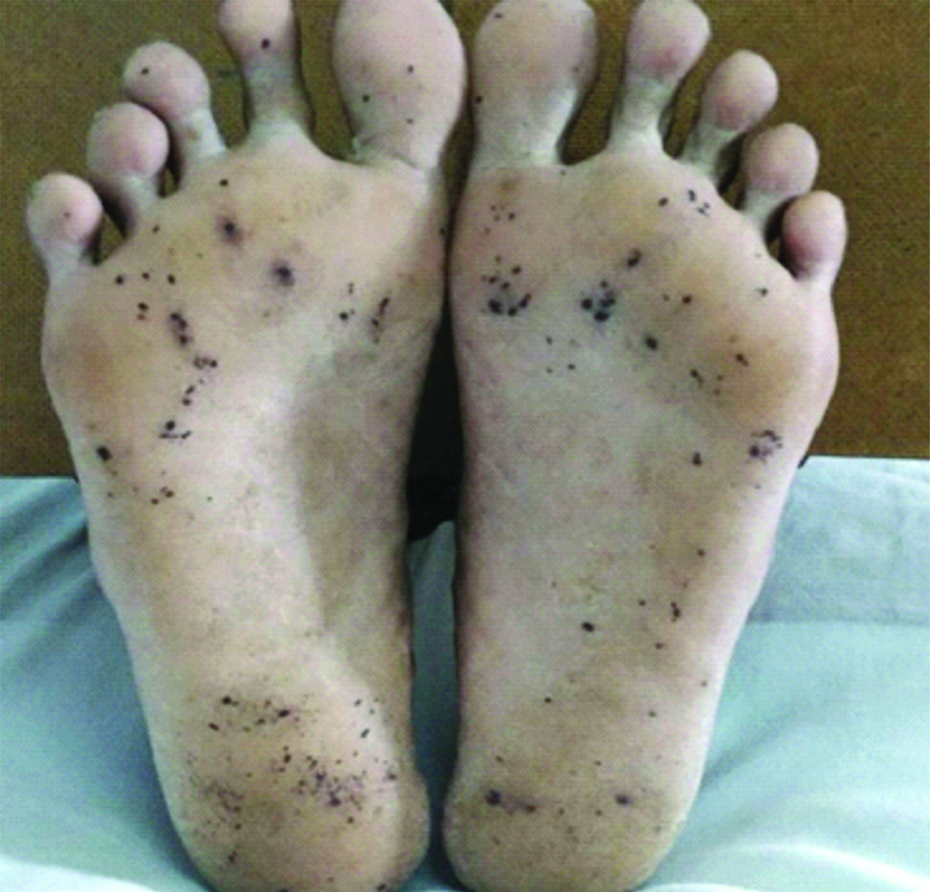
A 56-year-old male, presented with low-grade intermittent fever associated with generalised myalgia and loss of appetite for three months duration. No other associated co-morbid illness and no history of any cardiac disorders were present. He was a known alcoholic however, quit alcohol seven months ago and a non-smoker. On examination, patient was stable with a pulse rate of 106/minute, Blood Pressure (BP)-110/80 mmHg and 98% of oxygen saturation, afebrile, pallor present, Grade 3 clubbing and bilateral axillary lymphadenopathy were noted [Table/Fig-1]. Irregular, erythematous, flat, painless macules suggestive of Janeway lesions were seen on the soles and plantar surfaces of the toes [Table/Fig-2]. Auscultation of chest revealed pan-systolic murmur over the mitral area, tricuspid area and pulmonary area. Abdominal examination revealed hepatomegaly with a liver span of 15 cm, no signs of splenomegaly.
Demonstration of Grade 3 clubbing in finger nails.
Demonstration of Janeway lesions on the soles and plantar surface of toes.
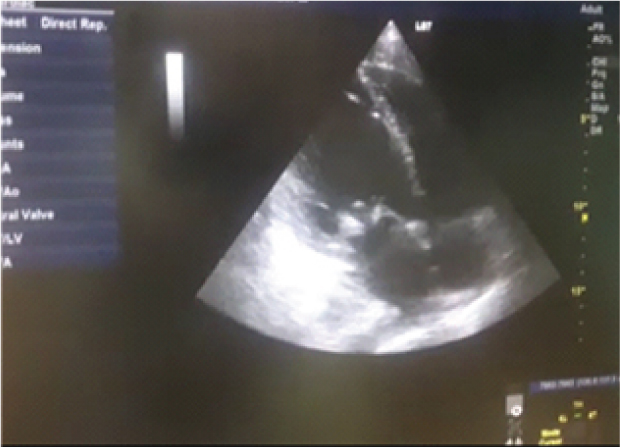
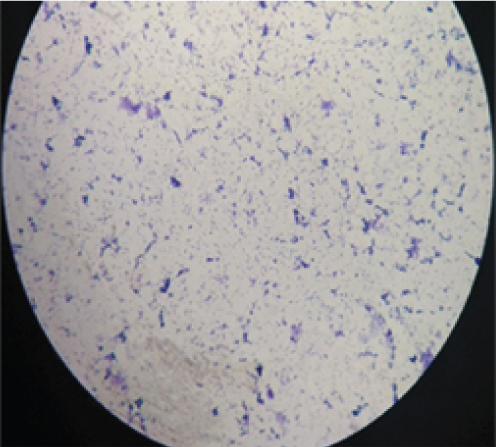
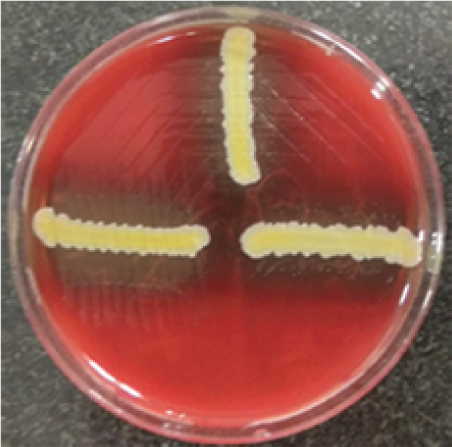
Routine investigations were done as per the admission protocol, and relevant report is as follows, haemoglobin-5 g/dL; total leucocyte count of 1800 cells/μL; serum creatinine-3.6 mg/dL; platelet count-50,000 cells/μL. Echocardiogram revealed multiple vegetations in the mitral leaflet, severe mitral regurgitation and moderate tricuspid regurgitation [Table/Fig-3]. Subsequently, a diagnosis of possible infective endocarditis was made according to the modified Duke’s criteria [1]. Three sets (two aerobic bottles per set) of blood culture samples (BACT/ALERT, BioMérieux) were drawn within two hours duration, before administering first dose of antibiotics. All six bottles flagged positive with a mean Time To Positivity (TTP) of six hours. Gram stain was performed directly from the blood culture bottles, which revealed Gram-positive cocci arranged in pairs and short chains [Table/Fig-4]. Subcultures were done on to Sheep Blood Agar (SBA) with perpendicular beta haemolytic Staphylococcus aureus streak on primary and secondary streak lines and Macconkey Agar (MAC). After overnight incubation, SBA showed minute, non-haemolytic opaque colonies with characteristic showed satellite colonies [Table/Fig-5]; MAC showed no growth (was used as part of routine blood culture protocol, absence of growth in MAC denotes the fastidious nature of organism). Culture smears showed gram-positive cocci arranged in pairs, short chains and coccobacillary forms. The isolate was catalase negative, PYR test positive and bile esculin test negative. For further precise identification, clinical isolate was subjected to Matrix Assisted Laser Desorption Ionisation-Time of Flight Mass Spectrometry (MALDI-TOFMS) by VITEK-MS (BioMérieux) and isolate was identified as Granulicatella adiacens. AST was not performed as the corresponding panel for NVS was not available for VITEK; authors were able to do identification only. Final clinical diagnosis was done as “Acute infective endocarditis due to NVS”. Patient was started on injection ceftriaxone two gram IV once daily and injection gentamycin 70 mg IV 24 hourly. Patient responded to the treatment and was symptomatically better at day five and repeat blood cultures sent on day five was negative. Treatment continued for a period of six weeks. Rest of the hospital stay was uneventful and the blood counts and ESR came down gradually. The patient was discharged after six weeks of admission and he was doing well on review after one month.
Demonstration of multiple vegetations in echocardiogram.
Gram stain of Granulicatella adiacens.
Blood agar demonstration of satellitism of Granulicatella adiacens.
Discussion
Infective Endocarditis is an uncommon but a life-threatening infectious disease with an annual incidence ranging from 3 to 7 per 100 000 person per years [2]. Major risk factors include cardiac valvular abnormalities, congenital heart diseases, prosthetic valves and drug abusers [3]. Viridans streptococci, Streptococcus gallolyticus (S bovis), HACEK group, S. aureus, enterococci are the typical organisms causing IE [4]. Approximately 3% to 5% of cases of streptococcal infective endocarditis is caused by nutritionally variant streptococci (NVS); also called as thiol-dependent or symbiotic streptococci [5]. Rate of complications is more for IE caused by NVS than other streptococci. Embolisation occurred in 27% and mortality rate was 17% to 20% because of severe heart failure [6]. The NVS has also been implicated in causing invasive infections, including abscess, wound infections, and meningitis [7].
Usually, IE due to NVS is frequently seen in cases with pre-existing valvular pathology. However, the present case is a case of native valve endocarditis [8]. Similar case is reported by Padmaja K et al., where IE due to NVS developed without any pre-existing cardiac abnormalities [6]. Treatment options for NVS causing IE includes combination therapy with penicillin and gentamycin (or vancomycin alone) for a duration of 4-6 weeks [9]. Performing Antimicrobial Susceptibility Testing (AST) remains a challenge, owing to the fastidious and slow-growing nature of the organism. According to EUCAST and CLSI recommendations, AST for NVS should be performed by microbroth dilution to determine Minimum Inhibitory Concentration (MIC) Some strains of NVS may exhibit a phenomenon of penicillin tolerance. For tolerants strains, the Minimum Bactericidal Concentration (MBC) for penicillin is around 32 times its MIC. These strains are more slowly inhibited by penicillin as found by one study on animal. Treatment failure may be seen in 41% of cases caused by NVS, among which 27% may require prosthetic valve replacement [8]. Slower generation time of these organisms adds to slower response of beta lactams. Slower generation further delays in isolation and identification of this group [10]. In a review research done by Giuliano S et al., in 2012 there were 22 documented cases of IE by NVS [9]. In this study, Granulicatella spp. was found in 57% of the cases and overall mortality rate was 9% among NVS endocarditis [10]. Jones BM et al., reported a case of penicillin intermediate and ceftriaxone resistant Granulicatella spp in 2017, and the patient was successfully treated with IV vancomycin for eight weeks [10]. However, in the present case patient responded well to a combination of beta lactam and aminoglycoside.
Conclusion
Infective Endocarditis caused by nutritionally variant streptococci may exhibit higher rates of relapse, treatment failure and morbidity than other streptococci. Early surgical intervention may be needed in penicillin-resistant cases. In the present case, the diagnosis was done within a time span of three days after admission and rigorous antibiotic treatment was started. Early prompt diagnosis and appropriate treatment prevented serious complications. High degree of suspicion combined with intensive laboratory workup in such cases may be lifesaving.
[1]. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Infective Endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart associationCirculation 2015 132:1435-86.10.1161/CIR.000000000000029626373316 [Google Scholar] [CrossRef] [PubMed]
[2]. Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, MinnesotaMayo Clin Proc 2010 85(5):422-26.10.4065/mcp.2009.058520435834 [Google Scholar] [CrossRef] [PubMed]
[3]. Heart Valves and Infective Endocarditis [Internet]. [cited 2018 Mar 13]. Available from: http://www.heart.org/HEARTORG/Conditions/More/HeartValveProblemsandDisease/Heart-Valves-and-Infective-Endocarditis_UCM_450448_Article.jsp#.WqdwwnzhXIW [Google Scholar]
[4]. Thuny F, Diagnostic Criteria for Infective Endocarditis. In: Infective Endocarditis Springer, Cham 2016 [cited 2018 Mar 13] :81-86.10.1007/978-3-319-32432-6_7 [Google Scholar] [CrossRef]
[5]. Lin C-H, Hsu R-B, Infective endocarditis caused by nutritionally variant streptococciAm J Med Sci 2007 334(4):235-39.10.1097/MAJ.0b013e3180a6eeab18030177 [Google Scholar] [CrossRef] [PubMed]
[6]. Padmaja K, Lakshmi V, Subramanian S, Neeraja M, Krishna SR, Satish OS, Infective endocarditis due to Granulicatella adiacens: a case report and reviewJ Infect Dev Ctries [Internet] 2014 Apr 15 [cited 2018 Mar 13] 8(04)10.3855/jidc.368924727523 [Google Scholar] [CrossRef] [PubMed]
[7]. Alberti MO, Hindler JA, Humphries RM, Antimicrobial Susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegansAntimicrob Agents Chemother 2016 60(3):1411-20.10.1128/AAC.02645-1526666926 [Google Scholar] [CrossRef] [PubMed]
[8]. Shailaja TS, Sathiavathy KA, Unni G, Infective endocarditis caused by Granulicatella adiacensIndian Heart J 2013 65:447-49.10.1016/j.ihj.2013.06.01423993006 [Google Scholar] [CrossRef] [PubMed]
[9]. Giuliano S, Caccese R, Carfagna P, Vena A, Falcone M, Venditti M, Endocarditis caused by nutritionally variant streptococci: a case report and literature reviewInfez Med 2012 20(2):67-74. [Google Scholar]
[10]. Jones BM, Hersey RM, Trestman IJ, Bland CM, Successful treatment of a penicillin-intermediate and ceftriaxone-resistant Granulicatella adiacens presumed prosthetic valve endocarditis with vancomycinInt J Antimicrob Agents 2018 51(3):508-10.10.1016/j.ijantimicag.2017.12.03129330034 [Google Scholar] [CrossRef] [PubMed]