An Interesting Case of Virilising Malignant Steroid Cell Tumour in a Post Menopausal Female: A Case Report
Shivanjali Raghuvanshi1, Vanshika Shahi2, Preeti Agarwal3, Sameer Gupta4, Madhu Mati Goel5
1 Assistant Professor, Department of Pathology, King George Medical University, Lucknow, Uttar Pradesh, India.
2 Senior Resident, Department of Pathology, King George Medical University, Lucknow, Uttar Pradesh, India.
3 Associate Professor, Department of Pathology, King George Medical University, Lucknow, Uttar Pradesh, India.
4 Associate Professor, Department of Surgical Oncology, King George Medical University, Lucknow, Uttar Pradesh, India.
5 Professor, Department of Pathology, King George Medical University, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shivanjali Raghuvanshi, Assistant Professor, Department of Pathology, King George Medical University, Lucknow-226003, Uttar Pradesh, India.
E-mail: joyousshivi@gmail.com
The present case is of a rare malignant steroid cell tumour which accounts for less than 0.1% of ovarian tumours. Symptoms of virilisation on presentation were noted and patient had an ovarian tumour which turned out to be malignant steroid cell tumour. The present case is unique because presentation in post menopausal age group is rare and the case being malignant is unusual.
Inhibin, Ovarian tumour, Premenopausal
Case Report
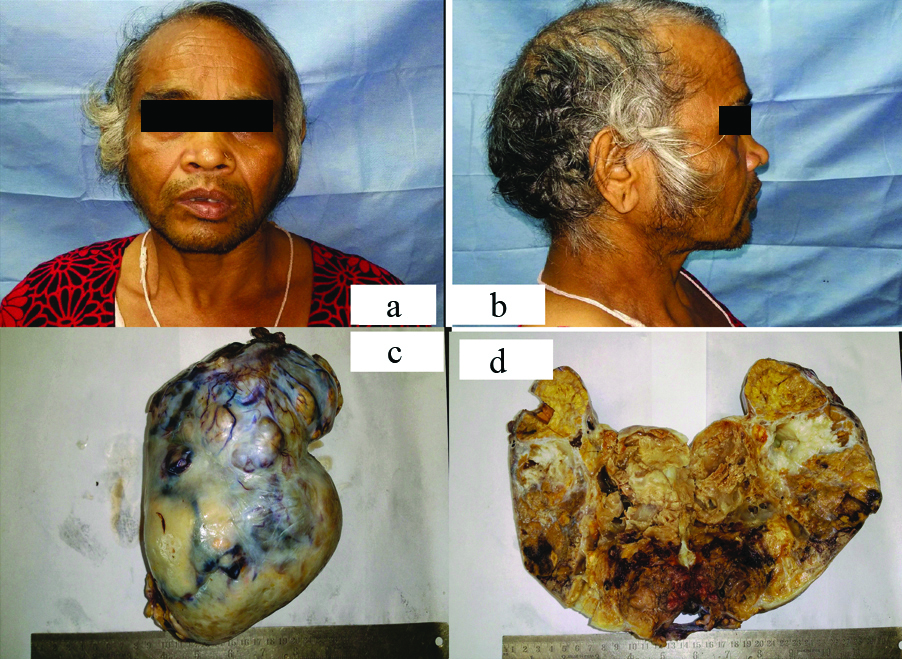
A 55-year-old post menopausal female presented in Outpatient Department of Surgical Oncology with symptoms of hirsutism, male pattern of baldness and change in character of voice since three years. She also complained of fullness and mass in abdomen since six months. She had history of diabetes mellitus and hypertension since six years. On physical examination, it was found that she had male body habitus with normal vital signs. She also had receding hairline, facial hair, prominent Adam’s apple, atrophied bilateral mammary glands and clitoral enlargement [Table/Fig-1a,b]. Hormonal investigations revealed serum total testosterone level of 2143 ng/dL (normal 30-95 ng/dL), follicle-stimulating hormone and luteinizing hormone values were 0.11 and 0.22 IU/L. Serum inhibin levels were 230.35 pg/mL (normal <2 pg/mL).
a,b) Clinical features of virilisation seen with coarse facial features, male pattern facial hair growth; c) Gross of ovary showing an encapsulated, bosselated left ovarian tumour; d) Cut surface showing a yellow tumour with areas of haemorhage and necrosis.
*Patient’s consent was taken before publishing the images.
Diagnosis and Treatment
Contrast enhanced computed tomography abdomen showed a 19.4×12.3×6.3 cm solid cystic lesion in left ovary with areas of necrosis and calcification. Rest of the organs were normal.
Patient underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy and supracolic omentectomy. Specimen was sent for histopathological examination.
A well encapsulated and bosselated left ovarian tumour was seen grossly. On cutting open, it was yellowish with areas of necrosis and haemorrhage [Table/Fig-1c,d].
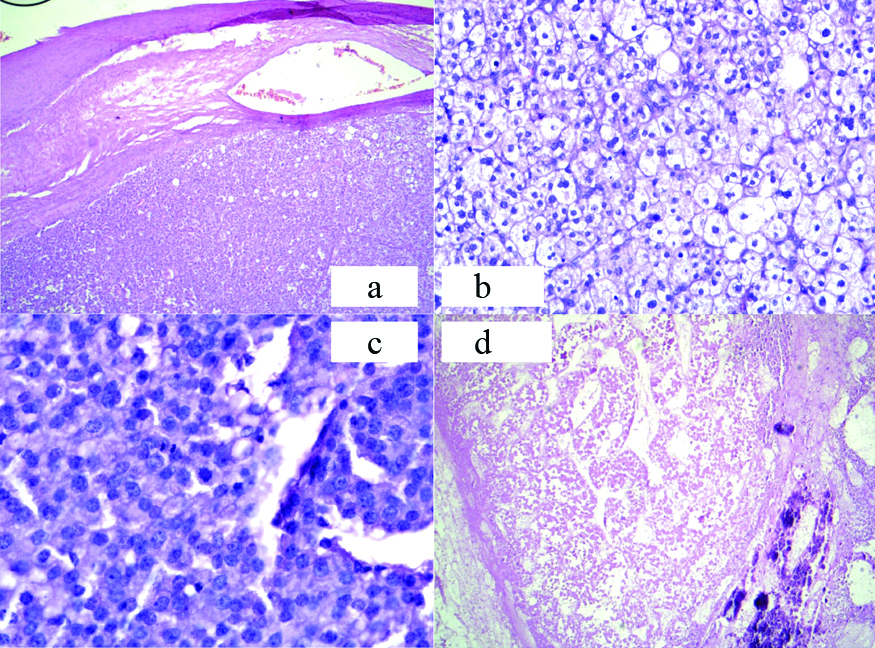
Microscopically the tumour cells were predominantly disposed in sheets. Tumour cells had centrally placed nucleus with abundant eosinophilic to clear cytoplasm. Occasional mitotic figures were noted (2-3/10 high power field). Few areas showed enlarged nucleus with coarse chromatin. Large areas of necrosis and calcification were also noted [Table/Fig-2].
a) Encapsulated tumour with well-defined capsule (H&E, 4X); b) Tumour cells had centrally placed nucleus with abundant eosinophilic to vacuolated cytoplasm (H&E, 20X); c) Occasional mitotic figures were noted (arrow) (H&E, 40X); d) Large areas of necrosis (thin arrow) and calcification (thick arrow) were noted (H&E, 4X).
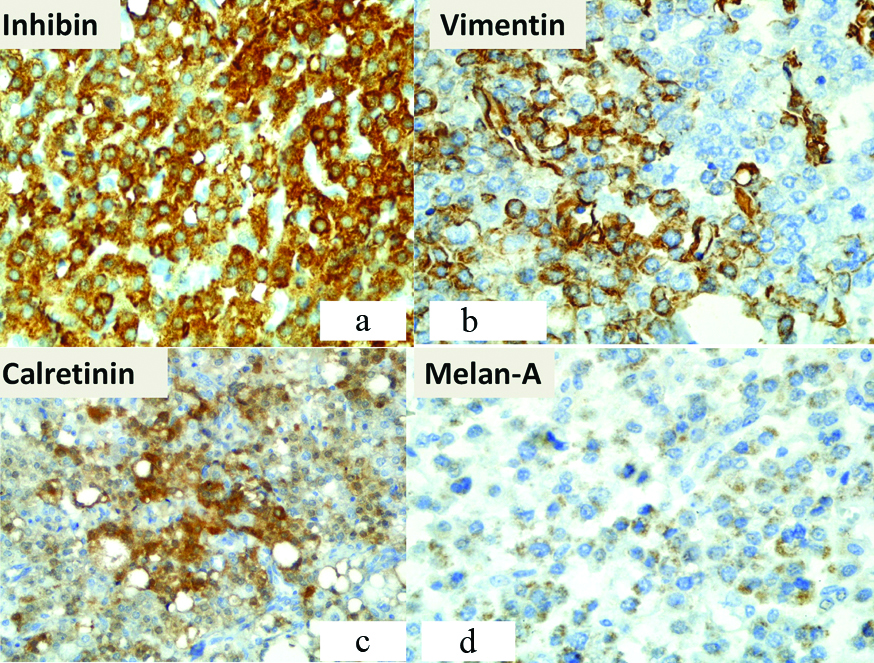
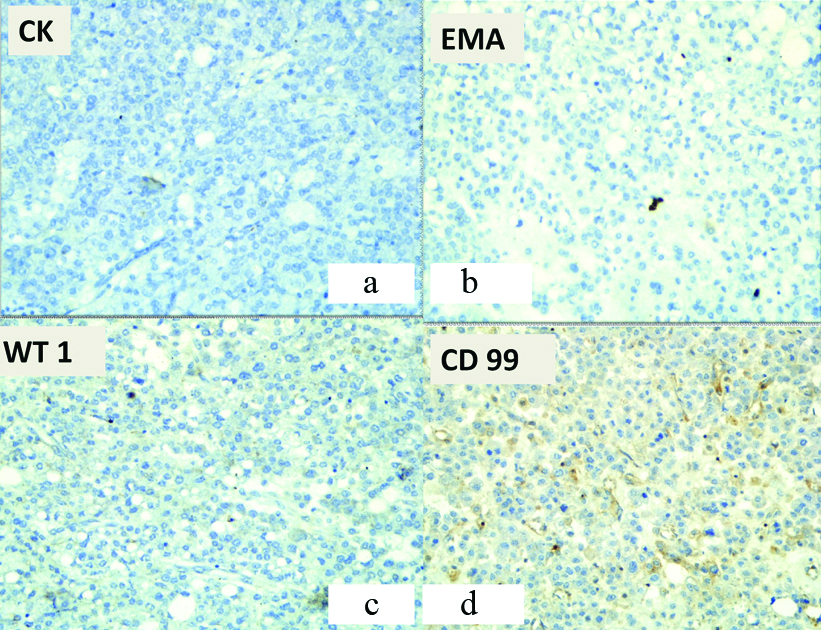
A differential diagnosis of extensively luteinized granulosa cell tumour, lipid cell sertoli cell tumour and steroid cell tumour (NOS subtype) was kept in mind and the following immunohistochemistry panel was applied revealing positivity for Inhibin-α (+++), Vimentin (++), Calretinin (++), Melanoma marker (Melan-A) (+) [Table/Fig-3]. Pan CK (-), EMA (-), WT-1 (-), CD99 (-) were negative [Table/Fig-4]. These findings indicated a malignant ovarian steroid cell tumour (NOS) and a diagnosis of left sided steroid cell tumour of the ovary was made with the pathological staging pT1aNxMx.
a) Immunohistochemistry for Inhibin was positive (+++) (Immunostain, 40X); b) Immunohistochemistry for vimentin was positive (++) (Immunostain, 40X); c) Immunohistochemistry for calretinin was positive (++) (Immunostain, 40X); d) Immunohistochemistry for melan-A was positive (+) (Immunostain, 40X).
a) Immunohistochemistry for Pan cytokeratin was negative (-) (Immunostain, 20X); b) Immunohistochemistry for EMA was negative (-) (Immunostain, 20X); c) Immunohistochemistry for WT 1 was negative (-) (Immunostain, 20X); d) Immunohistochemistry for CD 99 was negative (-) (Immunostain, 20X).
Discussion
Steroid cell tumour is described as ovarian tumours secreting steroid hormones. The classificationis based on the cell of origin-stromal luteoma when the tumour is arising from ovarian stroma, leydig cell tumour when it arises from Leydig cells in the hilus, and steroid cell tumour Not Otherwise Specified (NOS) when the origin of the tumour is not known [1].
Approximately 0.1% of ovarian tumours are steroid cell type out of which NOS type are one half [2], 25-45% of which are clinically malignant [3]. Oestrogen secretion occurs in 6% to 23% of the tumours, which may be associated with menorrhagia, post menopausal bleeding, or even endometrial adenocarcinoma. Around 6% to 10% of the cases present with Cushing’s syndrome. Hormonal disturbances have not been reported in approximately 25% cases of steroid cell tumour NOS type [2]. The average age of presentation is 43 years though these tumours have been reported to occur at any age.
Ultrastructurally, the abundant eosinophilic or vacuolated cytoplasm contains well-developed smooth endoplasmic reticulum and mitochondria with tubulovesicular cristae which stain positive for fat stains. Normal steroid hormone-secreting cells can be of lutein (thecal or stromal), leydig (hilus), and adrenal cortical type [4] and hence, it is important to keep the differentials in mind. On considering the diagnosis of steroid cell tumour NOS these differentials should also be kept in mind: stromal luteomas, leydig cell tumours, luteinized thecomas, luteinized granulosa cell tumours, pregnancy luteomas and carcinomas, primary clear cell carcinomas, metastatic renal cell carcinomas and adrenocortical carcinomas.
In the diagnosis of steroid cell tumours NOS, immunohistochemical staining of inhibin-α is generally useful. Regardless of their site of origin within the ovary, steroid cell tumours show strong cytoplasmic staining for inhibin, cytoplasmic and nuclear staining for calretinin, and nuclear staining for SF-1. Inhibin-α not only exists in supporting and stromal cells, but also in granulosa cells and luteal cells of the ovary. Particularly, among the pituitary gonadotropins, it is inhibited by follicle stimulating hormone. Steroid cell tumours differ from other types of sex cord-stromal tumours in that they rarely show immunostaining for Forkhead Box L2 (FOXL2) or Wilms Tumour-1 (WT-1) which are generally positive in sex cord tumours [5]. They typically show strong granular cytoplasmic staining for melan A. Recently, calretinin has been reported to be expressed by steroid cell tumours, NOS. It is a calcium binding protein present in nerve tissues, ovaries and testicles [6]. According to a report by Deavers MT et al., calretinin and inhibin-α expressions were found to be positive in 60% to 90% and in 5% to 90% of all steroid cell tumours NOS, respectively, and accordingly, in the present case, both inhibin-α and calretinin were positive upon immunohistochemistry [7]. In two-third cases, immunostains for androgen receptors are positive. Smooth Muscle Actin (SMA) is positive in about a third of cases. Hence, the best panel of stains to confirm the diagnosis is inhibin, calretinin, and SF-1; a negative Epithelial Membrane Antigen (EMA) helps exclude carcinoma.
Five pathologic features highly associated with malignancy given by Hayes and Scully were i) two or more mitoses per 10 high power fields; ii) a tumour diameter of >7 cm; iii) necrosis; iv) haemorrhage; and v) Grade 2 to 3 nuclear atypia [8]. Histologically, present case fulfilled four of these criteria except for the nuclear features.
Steroid cell tumour of the ovary being a rare entity, little focus has been assigned to its study. Most tumours are diagnosed at an early stage, they rarely recur or metastasise, hence the therapeutic value of chemotherapy and radiotherapy is poorly understood [9]. The most effective regimen is yet to be found, with the majority reporting poor efficacy. Recently, gonadotropin releasing hormone analogue has been used to treat this disease; gonadotropin releasing hormone agonist can inhibit the secretion of hormone [10].
Conclusion
To conclude ovarian steroid cell tumour, NOS are tumours of rare occurrence. Mostly are benign, unilateral and present with virilising symptoms. Pathological evaluation is essential for the diagnosis of malignancy, and immunohistochemical testing also aids in the formation of an accurate diagnosis. Surgery is the main treatment method and little is known about the response of the tumours to therapies such as chemotherapy or radiation. The present case is unique although the presentation is classic however, the case being malignant and in a post-menopausal age group is unusual and hence the case report.
[1]. Sood N, Desai K, Chindris AM, Lewis J, Dinh TA, Symptomatic ovarian steroid cell tumour not otherwise specified in a post-menopausal womanRare tumours 2016 8(2):620010.4081/rt.2016.620027441075 [Google Scholar] [CrossRef] [PubMed]
[2]. Amneus MW, Natarajan S, Pathologic quiz case: a rare tumour of the ovaryArch Pathol Lab Med 2003 127:890-92. [Google Scholar]
[3]. Jiang W, Tao X, Fang F, Zhang S, Xu C, Benign and malignant ovarian steroid cell tumours, not otherwise specified: case studies, comparison, and review of the literatureJournal of Ovarian Research 2013 6:5310.1186/1757-2215-6-5323870399 [Google Scholar] [CrossRef] [PubMed]
[4]. Rosai J, Ackerman’s Surgical Pathology 2011 10th EditionChicagoElsevier:1602 [Google Scholar]
[5]. Fletcher C, Crum C, Ayala A, Diagnostic Histopathology of Tumours 2013 4th EditionPhiladelphiaElsevier:703-05. [Google Scholar]
[6]. Kim JS, Park SN, Kim BR, Recurrent ovarian steroid cell tumours, not otherwise specified managed with debulking surgery, radiofrequency ablation, and adjuvant chemotherapyObstetrics & Gynecology Science 2014 57(6):534-38.10.5468/ogs.2014.57.6.53425469345 [Google Scholar] [CrossRef] [PubMed]
[7]. Deavers MT, Malpica A, Ordonez NG, Silva EG, Ovarian steroid cell tumours: an immunohistochemical study including a comparison of calretinin with inhibinInt J Gynecol Pathol 2003 22:162-67.10.1097/00004347-200304000-0000812649671 [Google Scholar] [CrossRef] [PubMed]
[8]. Hayes MC, Scully RE, Ovarian steroid cell tumours (not otherwise specified): a clinicopathological analysis of 63 casesAm J Surg Pathol 1987 11:835-45.10.1097/00000478-198711000-000022823622 [Google Scholar] [CrossRef] [PubMed]
[9]. Li K, Zhu F, Xiong J, Liu F, A rare occurrence of a malignant ovarian steroid cell tumour not otherwise specified: a case report and literature reviewOncology Letters 2014 8(2):770-74.10.3892/ol.2014.223325009655 [Google Scholar] [CrossRef] [PubMed]
[10]. Pascale MM, Pugeat M, Roberts M, Rousset H, Déchaud H, Dutrieux-Berger N, Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumoursClin Endocrinol (Oxf) 1994 41:571-76.10.1111/j.1365-2265.1994.tb01820.x7828344 [Google Scholar] [CrossRef] [PubMed]