Introduction
IFN-γ and IL-22 are Th1/Th17 cytokine which have been shown to play important role in the development of autoimmune disorder like psoriasis.
Aim
To identify the possible association of IFN-γ and IL-22 Single Nucleotide Polymorphisms (SNPs) with psoriasis as well as to find correlation between serum level of IL-22 and IFN-γ with severity of psoriasis.
Materials and Methods
A total of 168 psoriasis patients and 152 healthy controls were included in the study. A 3 ml of blood sample was collected from each psoriasis patients and healthy control for further analysis of SNP and serum level of cytokine. A part of blood sample was used for isolation of DNA for gene polymorphism study in psoriasis patients. Gene polymorphism of IL-22 (rs1179251 and rs2227513) and IFN-γ (rs2430561, rs2069709) were studied by ARMS PCR method whereas serum level of IL-22 and IFN-γ was analysed using commercially available ELISA kits. Odds Ratio (OR) with 95% Confidence Interval (95% CI) was calculated to assess the relative risk of psoriasis for various genetic variants.
Results
Serum level of IL-22 and IFN-γ was found to be (74.4±24.1 and 96.7±31.7 pg/ml respectively) significantly high (p<0.001), and associated with PASI score in psoriasis patients. In genotype analysis, a significant association was found between gene polymorphism of IFN-γ (rs2430561 and rs2069709) and IL-22 (rs1179251 and rs2227513) with psoriasis.
Conclusion
These results suggest that polymorphism of IFN-γ and IL-22 genes can be responsible for elevated serum level of IFN-γ and IL-22 cytokines. As these cytokines play key role in pathogenesis of psoriasis thus in conclusion gene polymorphism of IFN-γ and IL-22 may be responsible factor for increased susceptibility to psoriasis in north Indian population, Punjab, India.
Cytokines, Inflammatory, Interleukin, Polymorphism, Psoriasis
Introduction
Psoriasis is a chronic inflammatory skin disorder. External factors such as infection, stress, various drugs and alcohol can trigger an initial episode of psoriasis mainly in those individual who already have a genetic predisposition [1]. External stimulus activates the dendritic cells which starts migrating into the skin. Migrated dendritic cells starts priming of antigen specific T-cells. From here some T-cell and dendritic cell start to infiltrate the epidermis and causes the release of proinflammatory cytokines, which in turn stimulate keratinocyte proliferation [2]. Earlier it was considered as Th1/Th2 cytokine disease but recently some studies have confirmed the role of Th17 cytokine in the pathogenesis of psoriasis [3,4]. Th1/Th17 cytokine, IFN-γ and IL-22 have been shown to play an important role in the development of autoimmune disorders including psoriasis [5].
Among T cell-derived cytokines, IFN-γ is the most potent Th1 cytokine which activates the proinflammatory functions of keratinocytes. Along with that IFN-γ activated keratinocytes express a broad array of chemokines, cytokines, and membrane molecules that direct the recruitment, activation, and retention of specific leukocyte subpopulations in the skin [6,7]. Interleukin-22 (IL-22) is Th17 cytokine that has been reported recently to induce cutaneous inflammation in murine model of psoriasis. It can inhibit keratinocyte terminal differentiation and can induce epidermal alterations as appear in psoriasis [8]. There are such evidences that serum IL-22 levels is found to be increased in psoriatic patients and positively correlated with severity of disease [9]. Some other studies suggest that IL-22 mRNA was positively expressed in the psoriatic skin lesions, but negatively expressed in the normal controls [10].
IL22 is found to activate proliferative pathways in tissues, thus significantly controls tissue responses to inflammation. The role of IL-22 in inflammatory and autoimmune disorders remains controversial, with some studies suggesting a protective role, while others, a pathogenic role [11]. On the other hand, IL-22 acts as a mediator of cellular inflammatory responses by activating intracellular kinases as JAK1, Tyk2, and MAP kinases along with transcription factors such as STAT3 [12]. Furthermore, IL-22 exhibits anti-apoptotic and tumourigenic functions, with recent data showing that over-expression of that molecule protects lung cancer cell lines from apoptosis via activation of STAT3 and its downstream anti-apoptotic proteins Bcl-2 and Bcl-xL, and inactivation of extracellular signal-regulated kinases [13].
IFN-γ and IL-22 both are found to be involved in up-regulation of Interferon-inducible protein 16 (IFI16) in keratinocytes via activation of STAT3 signaling [14]. IFI16 activates TBK1-NF-κB signaling, leading to the production of CXCL10 and CCL20. Upregulation of IFI16 contributes to psoriasis by modulating chemokine production in keratinocytes [15].
So far it is very clear that psoriasis is characterised by increased production of cytokines. Key factors that affect the level of cytokine production may include post-translational modification, stability of intracellular protein and the export of cytokine to the extracellular environment. There are several studies which suggest that genetic polymorphisms of cytokine genes on promoter and protein encoding regions may contribute in all these factors thus, affecting the level of cytokine production in different individuals [16,17].
Being a signature cytokines of T helper (Th1/Th17) pathway, aim of the present study was to identify the correlation between serum level of IL-22 and IFN-γ with psoriasis and to establish a correlation between gene polymorphism of IL-22 and IFN-γ with psoriasis. Currently only few studies have reported that polymorphism of IL-22 and IFN-γ is associated with psoriasis [18,19]. Referring them, we conducted a case-control study to explore the possible association between IL-22 and IFN-γ Gene polymorphisms and development of psoriasis in north Indian population.
Materials and Methods
The present case control study was conducted among psoriasis patients visiting skin Outdoor Patients (OPD) of Dermatology Department of Government Hospitals and Medical Colleges of Punjab, India, between December, 2013 to November, 2016, whereas sample collection was completed till January, 2015.
Sample size was calculated on the basis of genotype and allele frequencies which we obtained in the pilot study, done on 25 subjects in each arm taking 5% level of significance at two tailed test, 80% power of the study (because there were no such studies available on such cases in that region). The difference of proportion of genotype frequencies varies for 0.14 to 0.19 for IL-22 and 0.18 to 0.22 for IFN-γ. The sample was calculated based on minimum difference of 0.18 which required 155 samples in each arm.
This study included freshly diagnosed psoriatic patient (prior to treatment) or psoriatic patients on treatment for topical (for two weeks), systemic and phototherapy (for four weeks). Whereas patient taking any alternative treatment (allopathic, ayurvedic, homeopathic) for psoriasis were excluded. Likewise psoriatic patient who were suffering from any other coexistent autoimmune disorders, acute or chronic infections, and malignancies were excluded from the study.
We screened 250 psoriatic patients visiting to skin outdoor patients from district hospitals of Punjab, India. They were screened following the inclusion and exclusion criteria. Out of 250 psoriatic patients 168 patients were recruited as cases for the study. A total of 152 out of 200 healthy volunteers, were recruited as control for the study. They were genetically-unrelated individuals. Patients above 18 years age, without any other infection as HIV, cancer or autoimmune diseases and taking no medications were included in the study. Individuals taking medicines for the treatment of psoriasis earlier than four weeks were excluded.
This study was approved by the ethics committee of Punjabi university Patiala, ICEC/2/2011. The methods were carried out in accordance with the approved guidelines and written informed consent was obtained from all participants.
Psoriasis Severity Assessment: Dermatological examination of all the psoriasis patients included in the study was performed. Assessment of psoriasis severity was done on the basis of Psoriasis Area and Severity Index (PASI) by the dermatologist [20].
Collection of Blood Sample A and Detection of Serum Cytokine: A 3 mL of venous blood from arms median cubital vein was, collected in sterile tube. A part of blood was further used for Genomic DNA isolation and another part was used for separation of serum. One ml venous blood samples were collected in sterile plane tube and was allowed to stand for 30 minutes at room temperature then centrifuged at 3000 rpm for 5 minutes. Sera was immediately separated and stored at -200°C for further analysis. Serum Cytokines were measured from serum samples of psoriatic patients and controls. Serum levels of IL-22 and IFN-γ were measured by Enzyme-Linked Immunosorbent Assay (ELISA) kits (KrishGen Biosystems, USA) according to the protocol.
SNP Selection and Genotyping: For the present study SNPs of IFN-γ and IL-22 genes were selected based on the following criteria: 1) minor allele frequency (MAF)≥ 0.05; 2) Hardy-Weinberg equilibrium test: P ≥ 0.05; and 3) SNPs located in the functional areas such as 5′ -UTR, 5′ near the gene, exon or 3′ -UTR. As a result, four SNPs were genotyped, including two SNPs each of IFN-γ (rs2430561 and rs2069709) and IL-22 (rs1179251 and rs2227513). Genomic DNA was extracted from peripheral blood sample by phenol/chloroform extraction method. PCR primers were designed by Web-based Allele Specific Primer designing tool (WASP). Genotyping was done using the ARMS PCR method. An internal control Human Growth Hormone (HGH) was amplified using a pair of primers designed from the nucleotide sequence of HGH in order to assess the success of PCR amplification [Table/Fig-1].
Primer sequences of IFN-γ and IL-22 alleles for specific PCR reaction.
| S.No. | Gene(SNP) | Primer sequences 5-----3 |
|---|
| 1 | IFN-γ T/Ars2430561 | Common Reverse Primer | 5′-TCA ACA AAG CTG ATA CTC CA-3′ |
| Forward Primer (T allele) | 5′-TTC TTA CAA CAC AAA ATC AAA TCT-3′ |
| Forward Primer (A allele) | 5′-TTC TTA CAA CAC AAA ATC AAA TCA-3′ |
| 2 | IFN-γ T/Grs2069709 | Common Reverse Primer | 5′-CTCTGGCTGCTGGTATTTAT-3′ |
| Forward Primer (T allele) | 5′-GGTCTGTCTCATCGTCAAGT-3′ |
| Forward Primer (G allele) | 5′-GGTCTGTCTCATCGTCAAGG-3′ |
| 3 | IL-22G/C(rs1179251) | Common Reverse Primer | 5′-GACTTCAAATAAATTTGCCC-3′ |
| Forward Primer (G allele) | 5′-TGTCTAGTCACATAACCTCGG-3′ |
| Forward Primer (C allele) | 5′-TGTCTAGTCACATAACCTCGC-3′ |
| 4 | IL-22 G/A(rs2227513) | A allele | 5′-CGTTTCGGCAAACTTGTTA-3′ |
| G allele | 5′-TTCATCAAACTAACCAATGGC-3′ |
| Forward Primer | 5′-CGTTTTAGGGAAACACTTGC-3′ |
| Reverse Primer | 5′-GTCCCCATAAGGAAAGAGCT-3′ |
Statistical Analysis
All statistical analyses were performed with Statistical Package for Social Sciences (SPSS, trial version 16.0). The deviations from Hardy–Weinberg Equilibrium (HWE) were determined using the Pearson χ2 test. Adjusted odds ratios (ORs) were calculated after correction for psoriasis risk factors with binary logistic regression with level of significance 5% and power of study 90%. The wild-type genotype/allele served as a reference category. A p-value <0.05 was considered significant.
Results
Clinical Profile of Psoriatic Patients: A total of 250 psoriatic patients were screened out of which 168 (67.2%) having 119 males and 49 females were found to be eligible and were included in the study. For the Control group, a total of 200 healthy individuals were screened and 152(76.0%) (106 Males and 46 females) were recruited to the study [Table/Fig-2]. Clinical characteristics of controls and patients are presented in [Table/Fig-2]. The mean age of cases and controls was 39.55±14.6 years and as 37.38±7.2 years respectively. In the study group, the mean PASI was calculated and found to be 15.1; moderate to severe psoriasis with PASI>10 was observed in 82 (48.8%) patients whereas moderate psoriasis was observed in 86 (51.2%) patients.
Characteristics of the study group vs. the control group.
| Characteristics | Patients (n=168) | Controls (n=152) |
|---|
| Mean±SD | Mean±SD |
|---|
| Age | 39.55±14.6 | 37.38±7.2 |
| Male (n) | 119 (70.83%) | 106 (69.73%) |
| Female (n) | 49 (29.17%) | 46 (30.27%) |
| Disease Duration (Years) | 8.68±8.2 | ----- |
| BMI(kg/m2) | 24.89±5.1 | 21.80±3.48 |
| PDI | 19.22±6.5 | ----- |
| PASI | 15.10±11.9 | ----- |
n: number of participants; SD- Standard Deviation; PASI: Psoriasis Area and Severity Index; PDI: Psoriasis Disability Index
Correlation between serum cytokine level of IFN-γ and IL-γ 22 with PASI: Serum levels of IFN-γ and IL-22 when measured in psoriatic patients they were found to be significantly high as compared with controls [Table/Fig-3a]. Likewise serum level of IL-22 as well as IFN-γ is positively correlated with PASI [Table/Fig-3b,c]. Detailed analysis of serum level of IL-22 and IFN-γ shows that there is no significant difference in serum level of IL-22 between mild to moderate psoriasis, where as there is a significant difference in serum level of IFN-γ between the same groups. Serum level of IFN-γ was found to be high i.e., 117.2±21.8pg/ml in cases having PASI ≥ 30 as compared to those having PASI ≤10 where it was found to be 56.6±11.8pg/ml. Similarly a significant difference was observed in serum level of IL-22 between psoriatic group having PASI ≤10 and ≥30 where values were observed as 59.4±13.3 and 84.6±20.7 respectively [Table/Fig-4].
Analysis of IL-16 levels in serum. The figure presents the cytokine serum levels: (a) in healthy controls and psoriasis patients; (b) Serum level of IL-22 in correlation with PASI; (c) Serum level of IFN-γ in correlation with PASI.
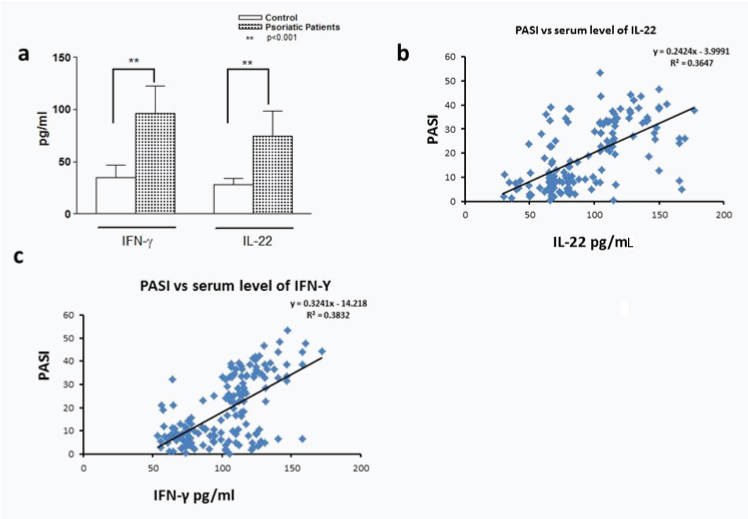
Serum level of IFN-γ and IL-22.
| Number of Cases/Control | IL-22 (pg/mL)Mean±SD | IFN γ (pg/mL)Mean±SD |
|---|
| Casegroup | 168 | 74.4±24.1 | 96.7±31.7 |
| Control group | 152 | 28.0±07.9 | 34.5±12.2 |
| Early-onset psoriasis | 97 | 66.6±14.9 | 90.3±27.6 |
| Late-onset psoriasis | 71 | 82.1±19.8 | 102.4±32.1 |
| Mild psoriasis | 86 | 79.3±23.3 | 64.9±23.7 |
| Moderate to severe psoriasis | 82 | 70.2±24.5 | 135.3±33.9 |
| PASI<=10 | 86 | 59.4±13.3 | 56.6±11.8 |
| 10<PASI>=20 | 42 | 75.6±21.6 | 73.4±24.7 |
| 20<PASI>=30 | 32 | 84.6±20.7 | 117.2±21.8 |
| PASI>30 | 08 | 83.7±33.9 | 153.4±18.9 |
SD: Standard Deviation; PASI: Psoriasis Area and Severity Index
Genotype Distribution and Allelic Frequencies of IFN-γ rs2430561 and rs2069709: The genotype distribution and allelic frequencies of the IFN-γ (rs2430561 and rs2069709) polymorphism in psoriasis patients and healthy controls are shown in [Table/Fig-5]. In this study all of the polymorphisms in psoriasis patients and controls were in HWE. As shown in results it was found that distribution of AA genotype and A allele of IFN-γ rs2430561 was found to be significantly higher in psoriasis patients than control as compared to TA genotype and T allele (OR = 2.5629, 95% CI: 1.519- 4.322, p<0.0001), whereas distribution of TG+TT genotypes together and T allele of IFN-γ rs2069709 was found to be higher in psoriasis patients as compared with TT genotype and G allele (OR=2.1236, 95% CI: 1.343-3.379, p<0.005).
Genotype Distribution and Allelic Frequencies of IL-22rs1179251and rs2227513: The GC genotype and C allele of IL-22rs1179251was found to be significantly higher in psoriasis patents than control as compared to GG genotype and G allele(OR = 2.7954, 95% CI: 1.615- 4.862, p<0.0001). Like-wise CC genotype was also found to be significantly higher in psoriasis patients (OR=2.332, 95% CI: 1.315- 4.134, p<0.005). We further observed that GA genotype and G allele of IL-22rs2227513 were found to be significantly higher in psoriasis patients than control as compared to AA genotype and A allele (OR =2.0277, 95% CI: 1.243-3.301(p<0.005). A significant association between GA genotype and G allele of IL-22rs2227513 and GC genotype and C allele of IL-22rs1179251 with psoriasis was observed from the results [Table/Fig-6].
Genotypes distribution and allele frequencies of the IFN-γ gene polymorphisms in psoriasis patients and controls.
| Genotype | Control (n=152) | % | Cases (n=168) | % | p | Adjusted OR*(95%CI)** |
|---|
| IFN-γ (rs2430561)T/A |
| TT | 78 | 51.3 | 50 | 29.8 | | Ref |
| TA | 32 | 21.1 | 49 | 29.2 | <0.005 | 2.3888(1.351-4.243) |
| AA | 42 | 27.6 | 69 | 41.1 | <0.0001 | 2.5629(1.519- 4.322) |
| TA+AA | 74 | 48.7 | 118 | 70.2 | <0.0001 | 2.4876(1.572-3.936) |
| T | 188 | 61.8 | 149 | 44.4 | | Ref |
| A | 116 | 38.2 | 187 | 55.6 | <0.0001 | 2.034(1.483-2.797) |
| IFN-γ (rs2069709) T/G |
| GG | 108 | 71.1 | 89 | 52.9 | | Ref |
| TG | 28 | 18.4 | 30 | 32.7 | <0.005 | 2.3836(1.397-4.068) |
| TT | 16 | 10.5 | 24 | 14.3 | >0.005 | 1.8202(0.921-3.616) |
| TG+TT | 44 | 28.9 | 77 | 45.8 | <0.005 | 2.1236(1.343-3.379) |
| G | 244 | 80.3 | 233 | 69.3 | | Ref |
| T | 60 | 19.7 | 103 | 30.7 | <0.005 | 1.7978(1.247-2.591) |
*Odds ratios (ORs) were obtained from a binary logistic regression model
**95 % CI, 95 % confidence interval
Genotypes distribution and allele frequencies of the IL-22 gene polymorphisms in psoriasis patients and controls.
| Genotype | Control (n=152) | % | Cases (n=168) | % | p | Adjusted OR*(95%CI)** |
|---|
| IL-22(rs1179251) |
| GG | 60 | 39.5 | 34 | 20.2 | | Ref |
| GC | 48 | 31.5 | 76 | 45.2 | <0.0001 | 2.7914(1.614-4.863) |
| CC | 44 | 28.9 | 58 | 34.5 | <0.005 | 2.3316(1.316- 4.131) |
| GC+CC | 92 | 60.5 | 134 | 79.8 | <0.0001 | 2.5743(1.575-4.233) |
| G | 168 | 55.3 | 144 | 42.9 | | |
| C | 136 | 44.7 | 192 | 57.1 | <0.005 | 1.6521(1.212- 2.253) |
| IL-22 (rs2227513) |
| AA | 77 | 50.7 | 60 | 35.7 | | Ref |
| GA | 50 | 32.9 | 79 | 47.0 | <0.005 | 2.0277(1.243-3.301) |
| GG | 25 | 16.4 | 29 | 17.3 | >0.005 | 1.4887(0.798-2.801) |
| GA+GG | 75 | 49.3 | 108 | 64.3 | <0.005 | 1.8481(1.181-2.892) |
| A | 204 | 67.1 | 137 | 40.8 | | |
| G | 100 | 32.9 | 199 | 59.2 | <0.0001 | 2.9625(2.153-4.092) |
*Odds Ratios (ORs) were obtained from a binary logistic regression model
**95 % CI, 95 % confidence interval
Discussion
It has been well identified that genetic background contributes to the clinical variability of psoriasis. Expression of many cytokines is thought to alter due to genetic polymorphism in their gene loci. In present work we have done a case-control study to identify the association between gene polymorphism of IFN-γ and IL-22 with psoriasis. Some previous studies have shown the role of IFN-γ in differentiation, proliferation, and effector function of Th1 cells. Likewise other studies have shown the role of IL-22 as Th17 effector cytokine. IFN-γ gene polymorphism was examined in many disorders liver cirrhosis, hashimoto disease, in the course of chronic hepatitis C [5,21]. There are few other studies which have reported the association between gene polymorphism of IFN-γ with psoriasis [18]. Here we have identified the association between IFN-γ (rs2430561 and rs2069709) gene polymorphism with psoriasis in Punjabi Population. IL-22 gene polymorphism was found to be associated with plaque psoriasis in Japanese population [19]. In another study 10 common polymorphisms of the IL-22 gene was identified in an Austrian population having plaque psoriasis but they were not found to be associated with psoriasis [22]. In reference to these previous studies we have identified association between IL-22 (rs1179251 and rs2227513) gene polymorphism with psoriasis in Punjabi population.
IFN-γ has been identified to induce psoriatic skin phenotype. Many studies have confirmed that serum levels of IFN-γ and the frequency of circulating CD8+IFN-γ+ T cells in the blood of patients is found to correlate with psoriasis disease severity [23-25]. In agreement with these findings we also reported here that serum level of IFN-γ is found to be significantly high and correlated with disease severity which is measured by PASI score. Although, IFN-γ has been shown to drive inflammation in skin, anti-proliferative effects on monolayer keratinocyte cultures yet its role needs to be examined in correct tissue context.
IL-22 and IFN-γ being signature cytokines of T helper Th1/Th17 cells, most of the studies have proven their role in psoriasis on the basis of expression level changes in mRNA [4,10,25]. It is found the allelic variants of genes for a high production of Th1 and Th2 cytokines might represent a risk factor for developing psoriasis. In agreement with that we reported here a possible correlation between IL-22 and IFN-γ gene polymorphism with pathogenesis of psoriasis. The current findings demonstrate that the frequency GC genotype and G allele of IL-22rs1179251 and GA genotype and A allele of IL-22 rs2227513 is greater in psoriatic patients. Likewise frequency of GC genotype and G allele of IL-22rs1179251 is also found to be greater in psoriatic patients. Some previous studies have reported that the single nucleotide polymorphism, T—A, at the 5′ end of the CA repeat of the human IFN-γ gene (+874T/A) directly affects the level of IFN-γ production and correlates with the presence of the A874 allele and low production of IFN-γ [26]. It is found that IFN-γ gene (+874T/A) polymorphism coincided with a putative nuclear factor κB(NF-κB) binding site that could have functional consequences for transcription of the human IFN-γ gene. There are studies which report that cytotoxic T lymphocytes are activated through NF-κB pathway [27]. All these studies suggest that the polymorphism could directly influence the level of IFN-γ production.
IL-22 is Th17 cytokines which is secreted under the induction of IL-17A, another Th17 cytokine. IL-17A activated via NF-κB pathway, upon increased secretion of IL17 it increases the secretion of IL-22 [28]. In agreement with this we also reported here that Serum level of IL-22 is found to be increased in psoriatic patients.
Limitation
This study was carefully done but still there were some limitations. The present study was confined only to Punjab, more states of the India be included. Although sample size for the study was chosen after statistical analysis yet its sample size was small. Therefore, future studies with large sample size are needed in different ethnic groups with carefully matched cases and controls to confirm the results of our findings.
Conclusion
In the present study, we found significant correlation between IL-22 and IFN-γ gene polymorphism with psoriasis in a North Indian population. There are many other gene polymorphism studies on IL-22 and IFN-γ gene, they may have discrepancies with current findings which may be due to differences in ethnicities, study design, and sample sizes. This is a small study, further large studies need to confirm these associations.
n: number of participants; SD- Standard Deviation; PASI: Psoriasis Area and Severity Index; PDI: Psoriasis Disability Index
SD: Standard Deviation; PASI: Psoriasis Area and Severity Index
*Odds ratios (ORs) were obtained from a binary logistic regression model
**95 % CI, 95 % confidence interval
*Odds Ratios (ORs) were obtained from a binary logistic regression model
**95 % CI, 95 % confidence interval
[1]. Takeshita J, Grewal S, Langan SM, Mehta Ogdie A, Van Voorhees Gelfand JM, Psoriasis and comorbid diseases: EpidemiologyJ Am Acad Dermatol 2017 76:377-90.10.1016/j.jaad.2016.07.06410.1016/j.jaad.2016.07.06428212759 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[2]. Karczewski J, Dobrowolska A, Rychlewska-Hanczewska A, Adamski Z, New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritisAutoimmunity 2016 46:435-50.10.3109/08916934.2016.116621427050731 [Google Scholar] [CrossRef] [PubMed]
[3]. Yousefzadeh H, Azad FJ, Rastin M, Banihashemi M, Mahmoudi M, Expression of Th1 and Th2 cytokine and associated transcription factors in peripheral blood mononuclear cells and correlation with disease severityReports of biochemistry & molecular biology 2017 6:102 [Google Scholar]
[4]. Arican O, Aral M, Sasmaz S, Ciragil P, Serum levels of TNF-α, IFN-α, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severityMediators of Inflammation 2005 5:273-79.10.1155/MI.2005.27316258194 [Google Scholar] [CrossRef] [PubMed]
[5]. Dai C-Y, Chuang W-L, Hsieh M-Y, Lee L-P, Hou N-J, Chen S-C, Polymorphism of interferon–gamma gene at position+ 874 and clinical characteristics of chronic hepatitis CTranslational Research 2006 148:128-33.10.1016/j.trsl.2006.04.00516938650 [Google Scholar] [CrossRef] [PubMed]
[6]. Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, De Pita O, IL-4 enhances keratinocyte expression of CXCR3 agonistic chemokinesJ Immunol 2000 165:1395-402.10.4049/jimmunol.165.3.139510903743 [Google Scholar] [CrossRef] [PubMed]
[7]. Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, Sozzani S, A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseasesJ Leukoc Biol 2001 70:617-23. [Google Scholar]
[8]. Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, A role for T cell-derived interleukin 22 in psoriatic skin inflammationClin Exp Immunol 2007 150:407-15.10.1111/j.1365-2249.2007.03511.x17900301 [Google Scholar] [CrossRef] [PubMed]
[9]. Shimauchi T, Hirakawa S, Suzuki T, Yasuma A, Majima Y, Tatsuno K, Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasisJ Dermatol 2016 40:805-12. [Google Scholar]
[10]. Mori H, Arita K, Yamaguchi T, Hirai M, Kurebayashi Y, Effects of Topical Application of Betamethasone on Imiquimod-induced Psoriasis-like Skin Inflammation in MiceKobe J Med Sci 2016 62:E79-E88. [Google Scholar]
[11]. Pinto LG, Talbot J, Peres RS, Franca RF, Ferreira SH, Ryffel B, Joint production of IL-22 participates in the initial phase of antigen-induced arthritis through IL-1 beta productionArthritis Res Ther 2015 17:015-0759.10.1186/s13075-015-0759-226330334 [Google Scholar] [CrossRef] [PubMed]
[12]. Nagalakshmi ML, Rascle A, Zurawski S, Menon S, Waal Malefyt RDe, Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cellsInt Immunopharmacol 2004 4:679-91.10.1016/j.intimp.2004.01.00815120652 [Google Scholar] [CrossRef] [PubMed]
[13]. Grivennikov SI, Karin M, Inflammation and oncogenesis: a vicious connectionCurrent Opinion in Genetics & Development 2010 20:65-71.10.1016/j.gde.2009.11.00420036794 [Google Scholar] [CrossRef] [PubMed]
[14]. Banno T, Adachi M, Mukkamala L, Blumenberg M, Unique keratinocyte-specific effects of interferon-gamma that protect skin from viruses, identified using transcriptional profilingAntiviral Therapy 2003 8:541-54. [Google Scholar]
[15]. Cao T, Shao S, Li B, Jin L, Lei J, Qiao H, Wang G, Up-regulation of Interferon-inducible protein 16 contributes to psoriasis by modulating chemokine production in keratinocytesSci Rep 2016 6:2538110.1038/srep2538127137868 [Google Scholar] [CrossRef] [PubMed]
[16]. Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV, In vitro production of IFN-gamma correlates with CA repeat polymorphism in the human IFN-gamma geneEur J Immunogenet 1999 26:1-3.10.1046/j.1365-2370.1999.00122.x10068907 [Google Scholar] [CrossRef] [PubMed]
[17]. Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV, An investigation of polymorphism in the interleukin-10 gene promoterEur J Immunogenet 1997 24:1-8.10.1111/j.1365-2370.1997.tb00001.x9043871 [Google Scholar] [CrossRef] [PubMed]
[18]. Baran W, Szepietowski JC, Mazur G, Baran E, IFN-gamma promoter gene polymorphism in psoriasis vulgarisBiomarkers 2008 13:52-58.10.1080/1354750070161027317852079 [Google Scholar] [CrossRef] [PubMed]
[19]. Saeki H, Hirota T, Nakagawa H, Tsunemi Y, Kato T, Shibata S, Genetic polymorphisms in the IL22 gene are associated with psoriasis vulgaris in a Japanese populationJ Dermatol Sci 2013 71:148-50.10.1016/j.jdermsci.2013.04.00223664643 [Google Scholar] [CrossRef] [PubMed]
[20]. Langley RG, Ellis CN, Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessmentJournal of the American Academy of Dermatology 2004 51:563-69.10.1016/j.jaad.2004.04.01215389191 [Google Scholar] [CrossRef] [PubMed]
[21]. Ito C, Watanabe M, Okuda N, Watanabe C, Iwatani Y, Association between the severity of Hashimoto’s disease and the functional+ 874A/T polymorphism in the interferon-γ geneEndocrine Journal 2006 53:473-78.10.1507/endocrj.K06-01516820703 [Google Scholar] [CrossRef] [PubMed]
[22]. Weger W, Hofer A, Wolf P, El-Shabrawi Y, Renner W, Kerl H, Common polymorphisms in the interleukin-22 gene are not associated with chronic plaque psoriasisExperimental Dermatology 2009 18:796-98.10.1111/j.1600-0625.2009.00840.x19469905 [Google Scholar] [CrossRef] [PubMed]
[23]. Johnson-Huang LM, Suarez-Farinas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skinJ Invest Dermatol 2012 132:1177-87.10.1038/jid.2011.45822277938 [Google Scholar] [CrossRef] [PubMed]
[24]. Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, IL-22–producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17–producing TH17 T cellsJournal of Allergy and Clinical Immunology 2009 123:1244-52. e2.10.1016/j.jaci.2009.03.04119439349 [Google Scholar] [CrossRef] [PubMed]
[25]. Ma H-L, Liang S, Li J, Napierata L, Brown T, Benoit S, IL-22 is required for Th17 cell–mediated pathology in a mouse model of psoriasis-like skin inflammationThe Journal of Clinical Investigation 2008 118:597-607.10.1172/JCI33263PMC2200300 [Google Scholar] [CrossRef] [PubMed]
[26]. Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AC, Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cellsJournal of Investigative Dermatology 2008 128:1207-11.10.1038/sj.jid.570121318200064 [Google Scholar] [CrossRef] [PubMed]
[27]. Yanan C, Haifeng L, Meifeng L, Shubin N, Jiaxin W, Hongwei S, Salvia miltiorrhiza polysaccharide activates T Lymphocytes of cancer patients through activation of TLRs mediated-MAPK and NF-kappaB Signaling PathwaysJ Ethnopharmacol 2017 20:31471-74. [Google Scholar]
[28]. Li ZJ, Choi DK, Sohn KC, Lim SK, Im M, Lee Y, Induction of Interleukin-22 (IL-22) production in CD4+ T Cells by IL-17A Secreted from CpG-Stimulated KeratinocytesAnn Dermatol 2016 28:579-85.10.5021/ad.2016.28.5.57927746637 [Google Scholar] [CrossRef] [PubMed]