Major Hepatectomies: Outcome and Perspectives from Eastern Nepal
Narendra Pandit1, Laligen Awale2, Shailesh Adhikary3
1 Associate Professor, Department of Surgery, BP Koirala Institute of Health Sciences, Dharan, Sunsari, Nepal.
2 Associate Professor, Department of Surgery, BP Koirala Institute of Health Sciences, Dharan, Sunsari, Nepal.
3 Professor, Department of Surgery, BP Koirala Institute of Health Sciences, Dharan, Sunsari, Nepal.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Narendra Pandit, Associate Professor, Department of Surgery, BP Koirala Institute of Health Sciences, Dharan-56700, Sunsari, Nepal.
E-mail: narendrapandit111@gmail.com
Introduction
Liver resection is a widely used surgical procedure for different benign and malignant pathology, which carries a significant morbidity and mortality (5-10%). Post-hepatectomy liver failure and bile leak are the major complications of major (>3 segments) hepatectomy, and is also the determinant factor for mortality. However, recently due to multimodality development like better surgeons experience, advanced imaging modality and better surgical planning, the outcome has significantly improved. Moreover, with relocation of experienced surgeons, selection of patients and sharing of operative techniques and perioperative care pathways had made possibility of performing major hepatectomy at even low volume academic centre, with almost comparable outcome to high volume centre.
Aim
To study the outcome of major hepatectomy at an academic institute of Nepal, which has a specialised hepatopancreatobiliary unit with its trained surgeon from a high volume centre.
Materials and Methods
Retrospective analysis of all patients undergoing major hepatectomy between December 2015 to July 2017 was done. Patient demographics, clinical characteristics, operative details, morbidity and mortality were recorded.
Results
There were 6 (24%) major hepatectomy, out of 25 liver resection. Five (83.3%) were for malignant pathology: Right hepatectomy-1; Extended right hepatectomy-1; Central hepatectomy-1; Left hepatectomy-3. The mean age of the patient was 50.8 years, with M:F ratio of 1:2. Jaundice was seen in 50% of patients and none required preoperative biliary drainage or portal vein embolisation. The mean operating time, blood loss and transfusion requirement were 216 minutes, 408 mL and one pint respectively. Three (50%) patients developed major bile leak, which was managed conservatively. There was no postoperative, 30-day or 90-day mortality.
Conclusion
Major hepatectomy is a safe and feasible option at our centre despite limited resources and low-volume set-up.
Liver resection, Low volume, Morbididty, Mortality
Introduction
Liver resection is a widely used surgical procedure for different benign and malignant pathology of liver [1]. According to Höhn’s classification [2], liver resection is classified as major abdominal surgery, which carries a significant morbidity and mortality. However, this procedure has markedly improved over the past three decades, with mortality rate more than 20% in 1970s to 10% in 1980s to recent reported rates below 5% [3,4].
Post-hepatectomy liver failure and bile leak is the major and dreaded complications of major liver resection and is also the determinant factor for the mortality [5-7]. However, present advancements permit such major procedure to be performed in low volume centres with outcomes comparable to high volume centres in affluent countries [8-10]. The academic institute (BP Koirala Institute of Health Sciences) has a specialised Hepatopancreatobiliary (HPB) unit established in 2015 with its trained surgeon from a high volume centre. This study documents the first successful series of major liver resections from a tertiary care academic centre in Eastern Nepal.
Materials and Methods
The present study was a retrospective analysis of all patients undergoing major hepatectomy (resection of three or more Couinaud segments) [11] for different benign and malignant liver disease at BP Koirala Institute of Health Sciences, Nepal. The institute is a 700 bedded hospital with its separate HPB and Gastrointestinal (GI) surgery division. From December 2015 until July 2017, all patients undergoing major hepatectomy in HPB unit were included. A written informed consent was obtained from all patients before surgery. All patients underwent the following to confirm the diagnosis and resectability of liver or biliary tract disease:
Complete detailed history and examination, including Body Mass Index (BMI) calculation, ASA score, comorbidities.
Laboratory tests: complete blood count, platelet count, creatinine, electrolytes, liver function test, albumin, total bilirubin, prothrombin time. All laboratory reports were calculated in relation to Child-Pugh’s score.
Imaging modalities: Chest X-ray, abdominal ultrasound, abdominal Contrast-Enhanced Computed Tomography (CECT), Magnetic Resonance Imaging/Cholangiopancreatography (MRI/MRCP).
Patients with technically resectable tumours of any origin with an estimated sufficient Future Liver Remnant (FLR) based on Child-Pugh’s score, platelet count, BMI and volume and quality on CECT/MRI were selected for major hepatectomy.
The variables studied included demographics, indication for surgery, need for preoperative biliary drainage, type of hepatic resections, duration of surgery, estimated blood loss, transfusion requirements, and any concomitant procedure along with hepatic resection. The type of liver resections performed was defined using the Brisbane 2000 nomenclature. Postoperative complications consistent with a Clavein-Dindo classification of surgical complication grade ≥IIIa were defined as morbidity. The “50-50” criteria was used to define Post-hepatectomy Liver Failure (PHLF), and the bile leak was graded as per International Study Group for Liver Surgery (ISGLS) [6]. Length of hospital stay, 30-day and 90-day mortality was also reviewed. Follow-up of patients was done by clinical examination, ultrasound/CECT abdomen every three to six months.
Statistical Analysis
Statistical analysis was performed in Microsoft Excel 2007 and results were presented as mean±SDs for normally distributed data and median and range for the rest of the variables.
Results
The study identified 6 (24%) adult patients out of 25 liver resections (major and minor) at BP Koirala Institute of Health Sciences, Sunsari, Nepal. The number of female patients was higher than that of male patients. The demographic profile, operative details and outcome of all the patients are summarised in [Table/Fig-1,2].
Patients demographics and clinical profile.
| Case | Age (years) | Sex | BMI (kg/m2) | ASA | CPS | Comorbidity | Total bilirubin (mg/dL) | Platelet count (×103/;L) | Obstructive Jaundice |
|---|
| 1 | 48 | M | 19.2 | II | A | no | 1.0 | 175 | no |
| 2 | 55 | F | 17.3 | II | A | no | 2.5 | 160 | yes |
| 3 | 50 | F | 18.2 | II | B | no | 17 | 140 | yes |
| 4 | 28 | M | 20 | III | A | no | 1.7 | 210 | no |
| 5 | 70 | F | 16.5 | II | B | DM | 20 | 155 | yes |
| 6 | 54 | F | 15.2 | II | A | no | 1.0 | 190 | no |
CPS: Child-pugh score; DM: Diabetes mellitus; BMI: Body mass index; ASA: American society of anaesthesiologists
Diagnosis, intraoperative details and outcome.
| Case | Diagnosis | Treatment | OT (min) | EBL (mL) | PRBT | Major morbidity | LOS (days) | Pathology | Follow-up* (months) |
|---|
| 1 | RPC | LH | 175 | 200 | no | no | 7 | RPC | Well (20) |
| 2 | GB cancer, Right portal pedicle involvement | ERH, BDE | 225 | 350 | no | Ascites | 14 | pT3N1M0R0 | Well (18) |
| 3 | HC (Bismuth-corlette Type IIIa) | RH, BDE | 230 | 410 | Yes (1 pint) | Bile leak (Grade B) | 22 | pT2N0M0R0 | Well (12) |
| 4 | UESL (intratumour bleeding) | CH | 240 | 620 | Yes (2 pint) | Ascites | 15 | pT3N0M0R1 | Recurrence (6) |
| 5 | HC (Bismuth corlette Type IIIb) | LH, BDE | 250 | 520 | Yes (1 pint) | Bile leak (Grade B) | 20 | pT2N1M0R0 | Well (8) |
| 6 | Intrahepatic cholangiocarcinoma | LH | 180 | 350 | Yes (1pint) | Bile leak (Grade B) | 9 | pT1b N0M0R0 | Well (6) |
RPC: Recurrent pyogenic cholangitis; GB: Gall bladder; HC: Hilar cholangiocarcinoma; LH: Left hepatectomy; ERH: Extended right hepatectomy; BDE: Bile duct excision; RH: Right hepatectomy; CH: Central hepatectomy; OT: Operative time; EBL: Estimated blood loss; PRBT: Packed red blood cell transfusion; LOS: Postoperative length of stay
*There were no post-hepatectomy liver failure, reoperation, readmission and 30 and 90-day mortality.
The mean age of the patients was 50.8 years. Three (50%) patients were having below normal BMI as per WHO classification with mean value of 17.7 kg/m2 [Table/Fig-3]. Five patients (83.3%) were in ASA 2 category and one patient was in ASA 3 category. The associated comorbidity (Type 2 diabetes mellitus) was present in one (16.6%) patient. Obstructive jaundice was seen in three patients (50%). Five patients (83.3%) required major hepatectomy for malignant cause [Table/Fig-4,5]. Concomitant bile duct excision with reconstruction was required in three patients (50%). None of the patients in present study was in acute cholangitis or severe malnutrition requiring preoperative biliary drainage/portal vein embolisation. Two patients requiring concomitant bile duct resection and one patient with left hepatectomy for intrahepatic cholangiocarcinoma developed bile leak (Grade B) in postoperative period, which was managed with radiological percutaneous drainage (Clavin-Dindo Grade III) [Table/Fig-2,3]. None of the patient developed post-hepatectomy liver failure despite few patients with high serum bilirubin (>10 mg%) undergoing upfront surgery. There was no reoperation, readmission or mortality for any complication. Two out of the five patients with malignant aetiology underwent postoperative adjuvant chemotherapy. After mean duration of 12.8 months follow-up of all patients are performing well with one patient having disease recurrence.
Baseline parameters and outcomes of major hepatectomy.
| Age (years, mean±SD) | 50.8±13.6 |
| Male:Female (M:F) | 1:2 |
| ASA-II | 5 (83.3) |
| ASA-III | 1 (16.6) |
| BMI (kg/m2) (mean±SD) | 17.7±1.7 |
| Comorbidity | |
| Diabetes mellitus, n (%) | 1 (16.6) |
| Obstructive jaundice, n (%) | 3 (50) |
| Total serum bilirubin (mg/dL) (median; range) | 2.1 (1 to 20) |
| Platelet count (×103/;L, mean±SD) | 171±25.4 |
| Pathology | |
| Benign, n (%) | 1 (16.6) |
| Malignant, n (%) | 5 (83.3) |
| Major Hepatectomy, n (%) | |
| Left hepatectomy (S2/3/4) | 3 (50) |
| Right hepatectomy (S5/6/7/8) | 1 (16.6) |
| Extended right hepatectomy (S4b/5/6/7/8) | 1 (16.6) |
| Central hepatectomy (S4/5/8) | 1 (16.6) |
| Combined resection of adjacent organs, bile duct excision, n (%) | 3 (50) |
| Operating time (minutes, mean±SD) | 216.6±31.5 |
| Estimated blood loss (mL, mean±SD) | 408.3±146.6 |
| Units of Packed red blood cell transfused (median) | 1 |
| Postoperative length of hospital stay (days, mean±SD) | 14.5±5.8 |
| Overall Major Morbidity, n (%) | 5 (83.3) |
| Bile leak (Grade B)- | 3 (50) |
| Ascites | 2 (33.3) |
| In hospital, 30 and 90-day mortality | nil |
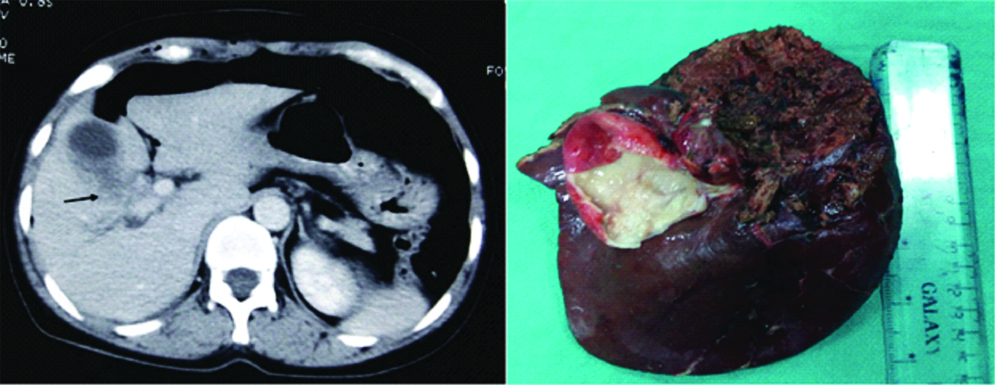
Computed Tomography (CT) scan (left) and intraoperative image (right) of patients with gallbladder cancer with right portal pedicle involvement (arrow) requiring extended right hepatectomy.
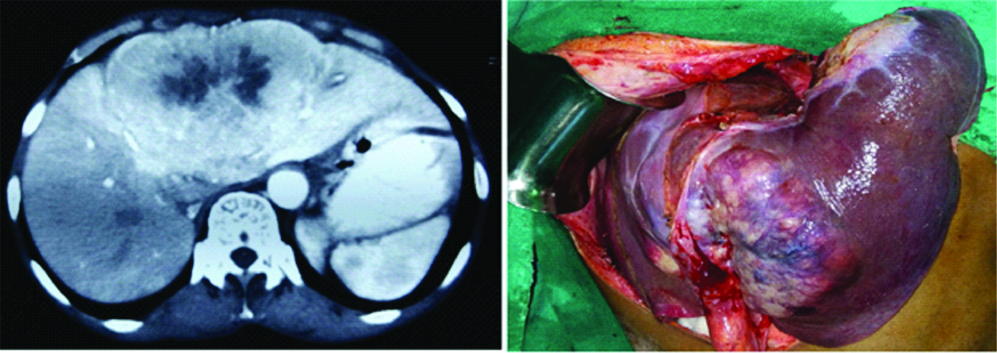
Computed Tomography (CT) scan (left) and intraoperative image (right) of patients with left liver intrahepatic cholangiocarcinoma requiring left hepatectomy.
Discussion
The present study is the first review of major hepatic resections in an Eastern Nepal and we have shown that this surgery is safe in terms of mortality/morbidity and is comparable to high-volume tertiary centres carrying out similar procedures [12,13].
Postoperative mortality has been well reported in large series from both low and high volume centres with available results consistently reporting inpatient and 90-day mortality rates of less than 5 to 8% [3]. Although the morbidity was high (83%), there were no mortality in present series, and the study clearly illustrates the safety of the procedure in our centre (BP Koirala Institute of Health Sciences, Sunsari, Nepal).
Bile leak is the most common complication and technical challenge to manage for surgeon after major hepatectomy. The reported incidence widely varies depending on the extent of hepatectomy and associated adjacent organ resection [14]. In one of the multi-institutional study, the proportion of patients developing bile leak was 8% after minor hepatectomy, more than 12% after major hepatectomy and the figure exceeded 30% after hepatectomy with bile duct reconstruction [15]. Bile leak after liver resection can lead to sepsis, organ space infection, prolonged hospital stay and cost, reoperation, readmission and a liver failure [16]. In the present case, we had 50% of patients with Grade B bile leak (Clavin-Dindo Class IIIa surgical complications) which was managed successfully by prolonged management of intraoperatively placed drain coupled with percutaneous radiological drainage of abdominal collection. This complication, although led to prolonged hospital stay, was devoid of any re-exploration and re-admission. The high incidence in present study could be presumed due to the small sample size and malignant pathology (hilar cholangiocarcinoma) requiring extrahepatic bile duct excision and reconstruction, a high risk group for bile leak.
Post-hepatectomy liver failure is the most dreaded complication after hepatectomy and is the major cause for increased morbidity, mortality and prolonged hospital stay. The reported incidence varies from 1% to 30%, and depends on the extent of liver resection, condition of liver, quality and quantity of the remnant liver and the intraoperative factors [6,17]. The PHLF increases in the presence of major hepatectomy in jaundiced patient, presence of portal hypertension (quantified by Child-pugh score and platelet count), associated comorbidity (diabetes mellitus) and increased intraoperative blood loss [18,19]. In the present study, there were no such complications, as none of the patients were having features suggestive of portal hypertension (low platelet count) affecting liver regeneration and all the patients were in child pugh Class A or early B. Moreover, the remnant volume was adequate for the proposed major hepatectomy, based on gross remnant liver volume on CT/MRI, BMI of the patient and associated comorbidity. Indeed it was the team proper patient selection, experience and “gut feeling” of probably patient not going to PHLF after the major resection [20].
The impact of volume-outcome effect has long been highlighted by Birkmeyer JD et al., for many surgical procedures [21]. The outcome of complex visceral procedures (pancreas, oesophagus, liver, lung and aorta) undergoing in high volume centre have been better than the low-volume procedures. However, recently, more and more studies have shown, almost similar outcome in low volume centre, compared to the high volume centre [9,22]. The improvement in mortality and morbidity cannot only be attributed to the volume effect. This has now been attenuated by the proper patient selection, surgeon experience, improvement in the perioperative and anaesthetic care, better understanding of the liver anatomy and establishment of separate skilled hepatobiliary surgical team [18,23]. Moreover, the mortality after major hepatectomy has also been due to “Failure To Rescue” (FTR) the major complication after surgery, a new metrics to assess the quality work at the institute [24]. A recent study by Ghaferi AA et al., from the University of Michigan, excellently depicted that the differences in mortality between high and low-volume cetres are not associated with large differences in complication rates. Instead, these differences seemed to be associated with the ability of a hospital to effectively rescue patients from complications [24,25]. The present study institute is a low-volume, academic institute with a dedicated surgical team and the system, where the residents, nurses and surgeons team are well versed with early detection and management of the complication, hence, mitigating the FTR and preventing the mortality.
Limitation
The major limitation of the studies was small sample size, failure to derive statistical influences and survival outcome.
Conclusion
Major hepatectomy is a safe and feasible option at our centre (BP Koirala Institute of Health Sciences) despite limited resources and low-volume set-up. The results in terms of morbidity and mortality were comparable to other larger series from international centres. However, further care should be taken to note the increased bile leak in the present study.
CPS: Child-pugh score; DM: Diabetes mellitus; BMI: Body mass index; ASA: American society of anaesthesiologists
RPC: Recurrent pyogenic cholangitis; GB: Gall bladder; HC: Hilar cholangiocarcinoma; LH: Left hepatectomy; ERH: Extended right hepatectomy; BDE: Bile duct excision; RH: Right hepatectomy; CH: Central hepatectomy; OT: Operative time; EBL: Estimated blood loss; PRBT: Packed red blood cell transfusion; LOS: Postoperative length of stay
*There were no post-hepatectomy liver failure, reoperation, readmission and 30 and 90-day mortality.
[1]. Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D’Angelica M, Harrison LE, Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinomaAnn Surg 2005 241(2):269-76.10.1097/01.sla.0000150244.72285.ad15650637 [Google Scholar] [CrossRef] [PubMed]
[2]. Al-Alem F, Mattar RE, Fadl OA, Alsharabi A, Al-Saif F, Hassanain M, Morbidity and mortality and predictors of outcome following hepatectomy at a Saudi tertiary care centerAnn Saudi Med 2016 36(6):414-21.10.5144/0256-4947.2016.41427920414 [Google Scholar] [CrossRef] [PubMed]
[3]. Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, Operative mortality after hepatic resection: Are literature-based rates broadly applicable?J Gastrointest Surg 2008 12(5):842-51.10.1007/s11605-008-0494-y18266046 [Google Scholar] [CrossRef] [PubMed]
[4]. Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: Analysis of 1222 consecutive patients from a prospective databaseIn: Annals of Surgery 2004 :698-710.10.1097/01.sla.0000141195.66155.0c15383797 [Google Scholar] [CrossRef] [PubMed]
[5]. Kawamura T, Noji T, Okamura K, Tanaka K, Nakanishi Y, Asano T, Postoperative liver failure criteria for predicting mortality after major hepatectomy with extrahepatic bile duct resectionDigestive Surgery 2018 10.1159/00048690629421802 [Google Scholar] [CrossRef] [PubMed]
[6]. Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: An international multicentre studyThe Official Journal of the International Hepato Pancreato Biliary Association 2018 20(5):462-69.Available from: https://www.ncbi.nlm.nih.gov/pubmed/2928773610.1016/j.hpb.2017.11.00729287736 [Google Scholar] [CrossRef] [PubMed]
[7]. Martin AN, Narayanan S, Turrentine FE, Bauer TW, Adams RB, Stukenborg GJ, Clinical factors and postoperative impact of bile leak after liver resection. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract.”J Gastrointest Surg 2018 22(4):661-67.Available from: https://www.ncbi.nlm.nih.gov/pubmed/2924742110.1007/s11605-017-3650-429247421 [Google Scholar] [CrossRef] [PubMed]
[8]. Al Hasan I, Tun-Abraham ME, Wanis KN, Garcia-Ochoa C, Levstik MA, Al-Judaibi B, Optimizing associated liver partition and portal vein ligation for staged hepatectomy outcomes: Surgical experience or appropriate patient selection?Can J Surg 2017 60(6):408-15.10.1503/cjs.00581729173259 [Google Scholar] [CrossRef] [PubMed]
[9]. Nygård IE, Lassen K, Kjæve J, Revhaug A, Mortality and survival rates after elective hepatic surgery in a low-volume centre are comparable to those of high-volume centresISRN Surg 2012 2012:78393210.5402/2012/78393222900204 [Google Scholar] [CrossRef] [PubMed]
[10]. Lee CW, Tsai HI, Sung CM, Chen CW, Huang SW, Jeng WJ, Risk factors for early mortality after hepatectomy for hepatocellular carcinomaMedicine (Baltimore) 2016 95(39):e5028Available from: https://www.ncbi.nlm.nih.gov/pubmed/2768487510.1097/MD.000000000000502827684875 [Google Scholar] [CrossRef] [PubMed]
[11]. Fan ST, Lo CM, Liu CL, Major hepatic resection for primary and metastatic tumours. In: Fischer, Josef EMastery of surgery 2007 5th editionLippincott Williams & Wilkins:1076-91. [Google Scholar]
[12]. Dimick JB, Pronovost PJ, Cowan J, Lipsett P, Postoperative complication rates after hepatic resection in Maryland hospitalsArch Surg 2003 138(1):41-46.10.1001/archsurg.138.1.4112511147 [Google Scholar] [CrossRef] [PubMed]
[13]. Dixon E, Bathe OF, McKay A, You I, Dowden S, Sadler D, Population-based review of the outcomes following hepatic resection in a Canadian health regionCan J Surg 2009 52(1):12-17. [Google Scholar]
[14]. Erdogan D, Busch ORC, Van Delden OM, Rauws EAJ, Gouma DJ, Van Gulik TM, Incidence and management of bile leakage after partial liver resectionDig Surg 2008 25(1):60-66.10.1159/00011802418292662 [Google Scholar] [CrossRef] [PubMed]
[15]. Hoekstra LT, Van Gulik TM, Gouma DJ, Busch OR, Posthepatectomy bile leakage: How to manageDig Surg 2012 29(1):48-53.10.1159/00033573422441620 [Google Scholar] [CrossRef] [PubMed]
[16]. Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, Bile leakage after hepatic resectionAnn Surg 2001 233(1):45-50.10.1097/00000658-200101000-0000811141224 [Google Scholar] [CrossRef] [PubMed]
[17]. Yadav K, Shrikhande S, Goel M, Post hepatectomy liver failure: concept of managementJournal of Gastrointestinal Cancer 2014 45:405-13.10.1007/s12029-014-9646-325104504 [Google Scholar] [CrossRef] [PubMed]
[18]. Russell MC, Complications following hepatectomySurgical Oncology Clinics of North America 2015 24:73-96.10.1016/j.soc.2014.09.00825444470 [Google Scholar] [CrossRef] [PubMed]
[19]. Au KP, Chan SC, Chok KSH, Chan ACY, Cheung TT, Ng KKC, Child-pugh parameters and platelet count as an alternative to ICG test for assessing liver function for major hepatectomyHPB Surg 2017 2017:294803010.1155/2017/294803028951631 [Google Scholar] [CrossRef] [PubMed]
[20]. Markus PM, Martell J, Leister I, Horstmann O, Brinker J, Becker H, Predicting postoperative morbidity by clinical assessmentBr J Surg 2005 92(1):101-06.10.1002/bjs.460815635697 [Google Scholar] [CrossRef] [PubMed]
[21]. Birkmeyer JD, Siewers AE, Finlayson EVA, Stukel TA, Lucas FL, Batista I, Hospital volume and surgical mortality in the United StatesN Engl J Med 2002 346(15):1128-37.10.1056/NEJMsa01233711948273 [Google Scholar] [CrossRef] [PubMed]
[22]. Kim SG, Paik KY, Noteworthy short term surgical outcomes following pancreatic resection by well trained surgeon at a low-volume institutionJournal of the Pancreas 2018 (I1):12-17. [Google Scholar]
[23]. Stella M, Bissolati M, Gentile D, Arriciati A, Impact of surgical experience on management and outcome of pancreatic surgery performed in high- and low-volume centersUpdates Surg 2017 69(3):351-58.10.1007/s13304-017-0422-328215039 [Google Scholar] [CrossRef] [PubMed]
[24]. Ghaferi AA, Birkmeyer JD, Dimick JB, Hospital volume and failure to rescue with high-risk surgeryMed Care 2011 49(12):1076-81.10.1097/MLR.0b013e3182329b9722002649 [Google Scholar] [CrossRef] [PubMed]
[25]. Toomey PG, Teta AF, Patel KD, Ross SB, Rosemurgy AS, High-volume surgeons vs high-volume hospitals: Are best outcomes more due to who or where?Am J Surg 2016 211(1):59-63.10.1016/j.amjsurg.2015.08.02126542187 [Google Scholar] [CrossRef] [PubMed]